Page 1114 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1114
It is a general result that the Woodward-Hoffmann rules predict that 1099
photochemical reactions will be complementary to thermal reactions. What is allowed
photochemically is forbidden thermally, and vice versa. The physical basis for this SECTION 12.2
complementary relationship is that the high barrier associated with forbidden thermal Photochemistry of
Alkenes, Dienes, and
reactions provides a point for strong interaction of the ground state and excited state Polyenes
species. As the two states are close in energy and of the same symmetry, they “mix”
and allow the excited molecule to reach the ground state. This interaction is necessary
for efficient photochemical reactions. 45
Let us now consider photochemical electrocyclic reactions. In Chapter 10, we
described the distinction between the conrotatory and disrotatory modes of reaction
as a function of the number of electrons in the system. Striking illustrations of
the relationship between orbital symmetry considerations and the outcome of photo-
chemical reactions are found in the stereochemistry of electrocyclic reactions. Orbital
symmetry predicts that photochemical electrocyclic reactions will show a reversal of
stereochemistry relative to thermal reactions. 46 One way of making this prediction is
to construct an electronic energy state diagram for the reactant and product molecule
and observe the correlation between the states. 47 The reactions in which the reacting
state correlates with a state of the product that is not appreciably higher in energy
are permitted. 48 The orbitals involved in the photochemical butadiene-to-cyclobutene
conversion are , , and of the first excited state of butadiene and , , and ∗
1 2 3
for cyclobutene. The appropriate elements of symmetry are the plane of symmetry for
the conrotatory and the axis of symmetry for the disrotatory process. The correlation
diagram for this reaction is shown in Figure 12.19. This analysis shows that disrotatory
1
2
cyclization is allowed, whereas conrotation would lead to a highly excited , , ∗1
configuration of cyclobutene. The same conclusion is reached if it is assumed that the
frontier orbital will govern reaction stereochemistry.
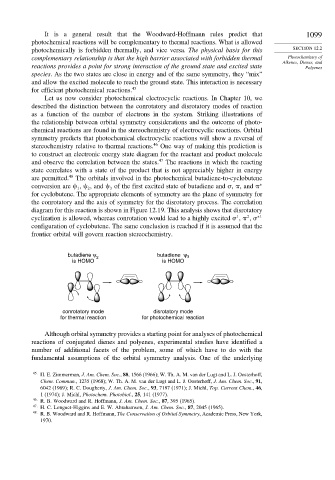
butadiene ψ 2 butadiene ψ 3
is HOMO is HOMO
conrotatory mode disrotatory mode
for thermal reaction for photochemical reaction
Although orbital symmetry provides a starting point for analyses of photochemical
reactions of conjugated dienes and polyenes, experimental studies have identified a
number of additional facets of the problem, some of which have to do with the
fundamental assumptions of the orbital symmetry analysis. One of the underlying
45 H. E. Zimmerman, J. Am. Chem. Soc., 88, 1566 (1966); W. Th. A. M. van der Lugt and L. J. Oosterhoff,
Chem. Commun., 1235 (1968); W. Th. A. M. van der Lugt and L. J. Oosterhoff, J. Am. Chem. Soc., 91,
6042 (1969); R. C. Dougherty, J. Am. Chem. Soc., 93, 7187 (1971); J. Michl, Top. Current Chem., 46,
1 (1974); J. Michl, Photochem. Photobiol., 25, 141 (1977).
46
R. B. Woodward and R. Hoffmann, J. Am. Chem. Soc., 87, 395 (1965).
47 H. C. Longuet-Higgins and E. W. Abrahamson, J. Am. Chem. Soc., 87, 2045 (1965).
48
R. B. Woodward and R. Hoffmann, The Conservation of Orbital Symmetry, Academic Press, New York,
1970.