Page 1115 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1115
1100
CHAPTER 12
Photochemistry
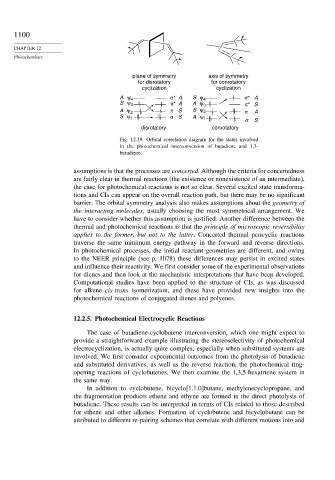
plane of symmetry axis of symmetry
for disrotatory for conrotatory
cyclization cyclization
A ψ 4 σ* A S ψ 4 σ* A
S ψ 3 π* A A ψ 3 π* S
A ψ 2 π S S ψ 2 π A
S ψ 1 σ S A ψ 1 σ S
disrotatory conrotatory
Fig. 12.19. Orbital correlation diagram for the states involved
in the photochemical interconversion of butadiene and 1,3-
butadiene.
assumptions is that the processes are concerted. Although the criteria for concertedness
are fairly clear in thermal reactions (the existence or nonexistence of an intermediate),
the case for photochemical reactions is not so clear. Several excited state transforma-
tions and CIs can appear on the overall reaction path, but there may be no significant
barrier. The orbital symmetry analysis also makes assumptions about the geometry of
the interacting molecules, usually choosing the most symmetrical arrangement. We
have to consider whether this assumption is justified. Another difference between the
thermal and photochemical reactions is that the principle of microscopic reversibility
applies to the former, but not to the latter. Concerted thermal pericyclic reactions
traverse the same minimum energy pathway in the forward and reverse directions.
In photochemical processes, the initial reactant geometries are different, and owing
to the NEER principle (see p. 1078) these differences may persist in excited states
and influence their reactivity. We first consider some of the experimental observations
for dienes and then look at the mechanistic interpretations that have been developed.
Computational studies have been applied to the structure of CIs, as was discussed
for alkene cis-trans isomerization, and these have provided new insights into the
photochemical reactions of conjugated dienes and polyenes.
12.2.5. Photochemical Electrocyclic Reactions
The case of butadiene-cyclobutene interconversion, which one might expect to
provide a straightforward example illustrating the stereoselectivity of photochemical
electrocyclization, is actually quite complex, especially when substituted systems are
involved. We first consider experimental outcomes from the photolysis of butadiene
and substituted derivatives, as well as the reverse reaction, the photochemical ring-
opening reactions of cyclobutenes. We then examine the 1,3,5-hexatriene system in
the same way.
In addition to cyclobutene, bicyclo[1.1.0]butane, methylenecyclopropane, and
the fragmentation products ethene and ethyne are formed in the direct photolysis of
butadiene. These results can be interpreted in terms of CIs related to those described
for ethene and other alkenes. Formation of cyclobutene and bicyclobutane can be
attributed to different re-pairing schemes that correlate with different motions into and