Page 1107 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1107
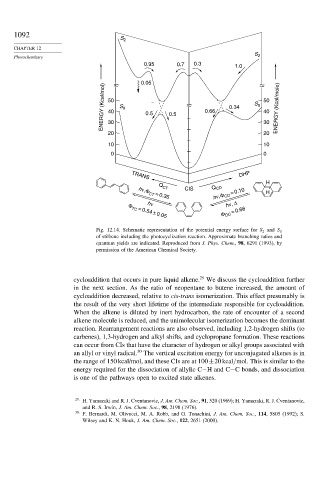
1092
S 2
CHAPTER 12
S
Photochemistry 2
0.95 0.7 0.3 1.0 ≈
≈
0.05
ENERGY (Kcal/mol) 50 S 0 0.5 0.5 ≈ 0.66 0.34 S 0 50 ENERGY (Kcal/mole)
40
40
30
20 30
20
10 10
0 0
DHP
TRANS
H
CIS Q CD
Q CT
hν,Φ CD = 0.10 H
hν,Φ CT = 0.35
h ν h ν, Δ
Φ DC = 0.66
Φ TC = 0.54 ± 0.05
Fig. 12.14. Schematic representation of the potential energy surface for S 2 and S 0
of stilbene including the photocyclization reaction. Approximate branching ratios and
quantum yields are indicated. Reproduced from J. Phys. Chem., 98, 6291 (1993), by
permission of the American Chemical Society.
29
cycloaddition that occurs in pure liquid alkene. We discuss the cycloaddition further
in the next section. As the ratio of neopentane to butene increased, the amount of
cycloaddition decreased, relative to cis-trans isomerization. This effect presumably is
the result of the very short lifetime of the intermediate responsible for cycloaddition.
When the alkene is diluted by inert hydrocarbon, the rate of encounter of a second
alkene molecule is reduced, and the unimolecular isomerization becomes the dominant
reaction. Rearrangement reactions are also observed, including 1,2-hydrogen shifts (to
carbenes), 1,3-hydrogen and alkyl shifts, and cyclopropane formation. These reactions
can occur from CIs that have the character of hydrogen or alkyl groups associated with
30
an allyl or vinyl radical. The vertical excitation energy for unconjugated alkenes is in
the range of 150 kcal/mol, and these CIs are at 100±20kcal/mol. This is similar to the
energy required for the dissociation of allylic C−H and C−C bonds, and dissociation
is one of the pathways open to excited state alkenes.
29 H. Yamazaki and R. J. Cventanovic, J. Am. Chem. Soc., 91, 520 (1969); H. Yamazaki, R. J. Cventanovic,
and R. S. Irwin, J. Am. Chem. Soc., 98, 2198 (1976).
30
F. Bernardi, M. Olivucci, M. A. Robb, and G. Tonachini, J. Am. Chem. Soc., 114, 5805 (1992); S.
Wilsey and K. N. Houk, J. Am. Chem. Soc., 122, 2651 (2000).