Page 1096 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1096
12.2. Photochemistry of Alkenes, Dienes, and Polyenes 1081
SECTION 12.2
We begin by discussing two fundamental types of photochemical reactions of
Photochemistry of
alkenes and dienes. One is cis-trans isomerization and the others fall into the category
Alkenes, Dienes, and
of pericyclic reactions, including electrocyclic reactions and cycloadditions. As Polyenes
indicated in Chapter 10, there is a broad dichotomy between thermal and photochemical
pericyclic reactions. Thermally forbidden processes are typically allowed photo-
chemically and vice versa. Although the interpretation and prediction of the stereo-
selectivity of pericyclic thermal reactions is generally possible within the framework
of the Woodward-Hoffmann rules, we will find several complicating factors when
we consider photochemical reactions. We also examine a number of unimolecular
photochemical rearrangements of alkenes and polyenes. Cycloadditions are considered
further, from a synthetic viewpoint, in Section 6.3.2 of Part B.
12.2.1. cis-trans Isomerization
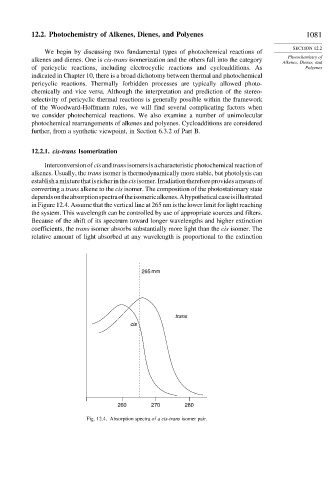
Interconversionofcisandtransisomersisacharacteristicphotochemicalreactionof
alkenes. Usually, the trans isomer is thermodynamically more stable, but photolysis can
establishamixturethatisricherinthecisisomer.Irradiationthereforeprovidesameansof
converting a trans alkene to the cis isomer. The composition of the photostationary state
dependsontheabsorptionspectraoftheisomericalkenes.Ahypotheticalcaseisillustrated
in Figure 12.4. Assume that the vertical line at 265 nm is the lower limit for light reaching
the system. This wavelength can be controlled by use of appropriate sources and filters.
Because of the shift of its spectrum toward longer wavelengths and higher extinction
coefficients, the trans isomer absorbs substantially more light than the cis isomer. The
relative amount of light absorbed at any wavelength is proportional to the extinction
265 mm
trans
cis
260 270 280
Fig. 12.4. Absorption spectra of a cis-trans isomer pair.