Page 1073 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1073
12 1057
TOPIC 11.2
log RATECONSTANT (calc.) 6 Reactions
Structure-Reactivity
9
Relationships in
Hydrogen Abstraction
3
0
0 3 6 9 12
log RATECONSTANT (exp.)
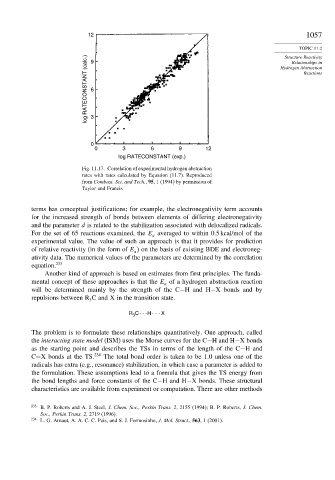
Fig. 11.17. Correlation of experimental hydrogen abstraction
rates with rates calculated by Equation (11.7). Reproduced
from Combust. Sci. and Tech., 95, 1 (1994) by permission of
Taylor and Francis
terms has conceptual justifications; for example, the electronegativity term accounts
for the increased strength of bonds between elements of differing electronegativity
and the parameter d is related to the stabilization associated with delocalized radicals.
For the set of 65 reactions examined, the E averaged to within 0.5 kcal/mol of the
a
experimental value. The value of such an approach is that it provides for prediction
of relative reactivity (in the form of E
on the basis of existing BDE and electroneg-
a
ativity data. The numerical values of the parameters are determined by the correlation
equation. 233
Another kind of approach is based on estimates from first principles. The funda-
mental concept of these approaches is that the E of a hydrogen abstraction reaction
a
will be determined mainly by the strength of the C−H and H−X bonds and by
repulsions between R C and X in the transition state.
3
R C H X
3
The problem is to formulate these relationships quantitatively. One approach, called
the interacting state model (ISM) uses the Morse curves for the C−H and H−X bonds
as the starting point and describes the TSs in terms of the length of the C−H and
C−X bonds at the TS. 234 The total bond order is taken to be 1.0 unless one of the
radicals has extra (e.g., resonance) stabilization, in which case a parameter is added to
the formulation. These assumptions lead to a formula that gives the TS energy from
the bond lengths and force constants of the C−H and H−X bonds. These structural
characteristics are available from experiment or computation. There are other methods
233 B. P. Roberts and A. J. Steel, J. Chem. Soc., Perkin Trans. 2, 2155 (1994); B. P. Roberts, J. Chem.
Soc., Perkin Trans. 2, 2719 (1996).
234
L. G. Arnaut, A. A. C. C. Pais, and S. J. Formosinho, J. Mol. Struct., 563, 1 (2001).