Page 1068 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1068
1052 The mechanism is the same as for aryl halides, but the dissociation of the halide is
probably concerted with the electron transfer.
CHAPTER 11
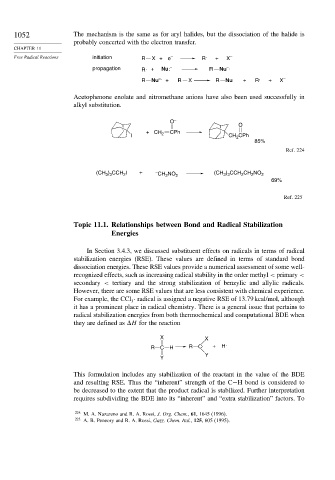
Free Radical Reactions initiation R X +e – R . + X –
propagation R . + Nu: – R Nu .
–
R Nu –. + R X R Nu + R . + X –
Acetophenone enolate and nitromethane anions have also been used successfully in
alkyl substitution.
O –
O
+ CH CPh
I 2 CH CPh
2
85%
Ref. 224
(CH ) CCH I + – CH NO 2 (CH ) CCH CH NO 2
3 3
2
2
2
3 3
2
69%
Ref. 225
Topic 11.1. Relationships between Bond and Radical Stabilization
Energies
In Section 3.4.3, we discussed substituent effects on radicals in terms of radical
stabilization energies (RSE). These values are defined in terms of standard bond
dissociation energies. These RSE values provide a numerical assessment of some well-
recognized effects, such as increasing radical stability in the order methyl < primary <
secondary < tertiary and the strong stabilization of benzylic and allylic radicals.
However, there are some RSE values that are less consistent with chemical experience.
For example, the CCl · radical is assigned a negative RSE of 13 79 kcal/mol, although
3
it has a prominent place in radical chemistry. There is a general issue that pertains to
radical stabilization energies from both thermochemical and computational BDE when
they are defined as H for the reaction
X X
R C H R C . + H .
Y
Y
This formulation includes any stabilization of the reactant in the value of the BDE
and resulting RSE. Thus the “inherent” strength of the C−H bond is considered to
be decreased to the extent that the product radical is stabilized. Further interpretation
requires subdividing the BDE into its “inherent” and “extra stabilization” factors. To
224 M. A. Nazareno and R. A. Rossi, J. Org. Chem., 61, 1645 (1996).
225
A. B. Peneory and R. A. Rossi, Gazz. Chem. Ital., 125, 605 (1995).