Page 1056 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 1056
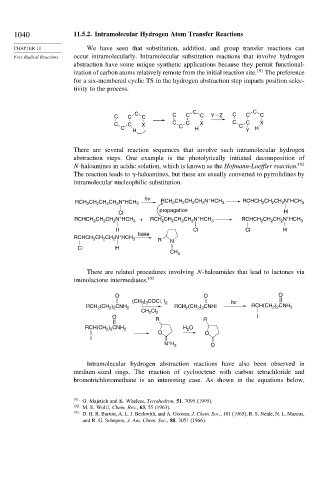
1040 11.5.2. Intramolecular Hydrogen Atom Transfer Reactions
CHAPTER 11 We have seen that substitution, addition, and group transfer reactions can
Free Radical Reactions occur intramolecularly. Intramolecular substitution reactions that involve hydrogen
abstraction have some unique synthetic applications because they permit functional-
ization of carbon atoms relatively remote from the initial reaction site. 191 The preference
for a six-membered cyclic TS in the hydrogen abstraction step imparts position selec-
tivity to the process.
C C C C C C C
C C C C C Y – Z
C C X . C C C . X C C C X
C H H
H Y
There are several reaction sequences that involve such intramolecular hydrogen
abstraction steps. One example is the photolytically initiated decomposition of
N-haloamines in acidic solution, which is known as the Hofmann-Loeffler reaction. 192
The reaction leads to -haloamines, but these are usually converted to pyrrolidines by
intramolecular nucleophilic substitution.
+
+
+ hv CH CH CH N HCH RCHCH CH CH N HCH
RCH CH CH CH N HCH 3 RCH 2 2 2 2 . 3 . 2 2 2 3
2
2
2
2
propagation
Cl H
+
+
+
RCHCH CH CH N HCH 3 + RCH CH CH CH N HCH 3 RCHCH CH CH N HCH 3
.
2
2
2
2
2
2
2
2
2
2
H base Cl Cl H
+
RCHCH CH CH N HCH 3 R N
2
2
2
Cl H
CH 3
There are related procedures involving N-haloamides that lead to lactones via
iminolactone intermediates. 193
O O O
) COCl, I
(CH 3 3 2 hv
2 2
RCH 2 (CH 2 ) 2 CNH 2 RCH 2 (CH 2 ) 2 CNHI RCH(CH ) CNH 2
CH Cl 2
2
O I
R R
RCH(CH ) CNH 2 H O
2
2 2
O O
I
+
N H 2 O
Intramolecular hydrogen abstraction reactions have also been observed in
medium-sized rings. The reaction of cyclooctene with carbon tetrachloride and
bromotrichloromethane is an interesting case. As shown in the equations below,
191
G. Majetich and K. Wheless, Tetrahedron, 51, 7095 (1995).
192 M. E. Wolff, Chem. Rev., 63, 55 (1963).
193
D. H. R. Barton, A. L. J. Beckwith, and A. Goosen, J. Chem. Soc., 181 (1965); R. S. Neale, N. L. Marcus,
and R. G. Schepers, J. Am. Chem. Soc., 88, 3051 (1966).