Page 25 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 25
4
CHAPTER 1
Chemical Bonding
and Molecular Structure
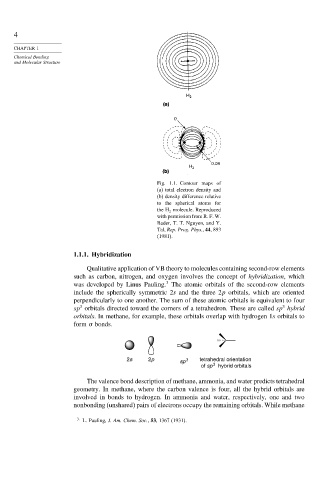
Fig. 1.1. Contour maps of
(a) total electron density and
(b) density difference relative
to the spherical atoms for
the H 2 molecule. Reproduced
with permission from R. F. W.
Bader, T. T. Nguyen, and Y.
Tal, Rep. Prog. Phys., 44, 893
(1981).
1.1.1. Hybridization
Qualitative application of VB theory to molecules containing second-row elements
such as carbon, nitrogen, and oxygen involves the concept of hybridization, which
3
was developed by Linus Pauling. The atomic orbitals of the second-row elements
include the spherically symmetric 2s and the three 2p orbitals, which are oriented
perpendicularly to one another. The sum of these atomic orbitals is equivalent to four
3
3
sp orbitals directed toward the corners of a tetrahedron. These are called sp hybrid
orbitals. In methane, for example, these orbitals overlap with hydrogen 1s orbitals to
form bonds.
2s 2p 3 tetrahedral orientation
sp 3
of sp hybrid orbitals
The valence bond description of methane, ammonia, and water predicts tetrahedral
geometry. In methane, where the carbon valence is four, all the hybrid orbitals are
involved in bonds to hydrogen. In ammonia and water, respectively, one and two
nonbonding (unshared) pairs of electrons occupy the remaining orbitals. While methane
3
L. Pauling, J. Am. Chem. Soc., 53, 1367 (1931).