Page 28 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 28
It is worth noting at this point that a particular hybridization scheme does not 7
provide a unique description of molecular structure. The same fundamental conclu-
sions about geometry and electron density are reached if ethene and ethyne are SECTION 1.1
3
described in terms of sp hybridization. In this approach, the double bond in ethene is Description of Molecular
Structure Using Valence
3
thought of as arising from two overlapping sp orbitals. The two bonds are equivalent Bond Concepts
and are called bent bonds. This bonding arrangement also predicts a planar geometry
and elliptical electron distribution, and in fact, this description is mathematically equiv-
2
alent to the sp hybridization description. Similarly, ethyne can be thought of as arising
3
by the sharing of three sp hybrid orbitals. The fundamental point is that there is
a single real molecular structure defined by atomic positions and electron density.
Orbitals partition the electron density in specific ways, and it is the sum of the orbital
contributions that describes the structure.
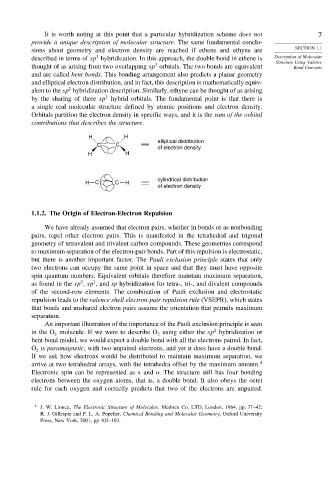
H H
elliptical distribution
C C
of electron density
H H
cylindrical distribution
H C C H
of electron density
1.1.2. The Origin of Electron-Electron Repulsion
We have already assumed that electron pairs, whether in bonds or as nonbonding
pairs, repel other electron pairs. This is manifested in the tetrahedral and trigonal
geometry of tetravalent and trivalent carbon compounds. These geometries correspond
to maximum separation of the electron-pair bonds. Part of this repulsion is electrostatic,
but there is another important factor. The Pauli exclusion principle states that only
two electrons can occupy the same point in space and that they must have opposite
spin quantum numbers. Equivalent orbitals therefore maintain maximum separation,
3
2
as found in the sp , sp , and sp hybridization for tetra-, tri-, and divalent compounds
of the second-row elements. The combination of Pauli exclusion and electrostatic
repulsion leads to the valence shell electron-pair repulsion rule (VSEPR), which states
that bonds and unshared electron pairs assume the orientation that permits maximum
separation.
An important illustration of the importance of the Pauli exclusion principle is seen
2
in the O molecule. If we were to describe O using either the sp hybridization or
2
2
bent bond model, we would expect a double bond with all the electrons paired. In fact,
O is paramagnetic, with two unpaired electrons, and yet it does have a double bond.
2
If we ask how electrons would be distributed to maintain maximum separation, we
arrive at two tetrahedral arrays, with the tetrahedra offset by the maximum amount. 4
Electronic spin can be represented as x and o. The structure still has four bonding
electrons between the oxygen atoms, that is, a double bond. It also obeys the octet
rule for each oxygen and correctly predicts that two of the electrons are unpaired.
4
J. W. Linnett, The Electronic Structure of Molecules, Methren Co. LTD, London, 1964, pp. 37–42;
R. J. Gillespie and P. L. A. Popelier, Chemical Bonding and Molecular Geometry, Oxford University
Press, New York, 2001, pp 102–103.