Page 32 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 32
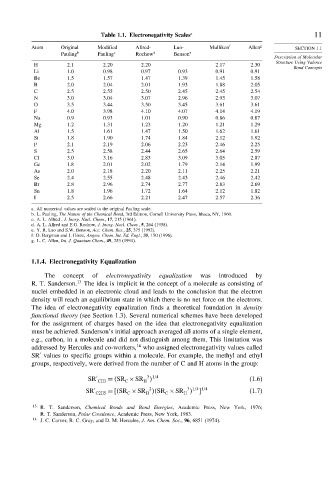
Table 1.1. Electronegativity Scales a 11
Atom Original Modified Allred- Luo- Mulliken f Allen g SECTION 1.1
Pauling b Pauling c Rochow d Benson e
Description of Molecular
Structure Using Valence
H 2 1 2 20 2 20 2 17 2 30
Bond Concepts
Li 1 0 0 98 0 97 0 93 0 91 0 91
Be 1 5 1 57 1 47 1 39 1 45 1 58
B 2 0 2 04 2 01 1 93 1 88 2 05
C 2 5 2 55 2 50 2 45 2 45 2 54
N 3 0 3 04 3 07 2 96 2 93 3 07
O 3 5 3 44 3 50 3 45 3 61 3 61
F 4 0 3 98 4 10 4 07 4 14 4 19
Na 0 9 0 93 1 01 0 90 0 86 0 87
Mg 1 2 1 31 1 23 1 20 1 21 1 29
Al 1 5 1 61 1 47 1 50 1 62 1 61
Si 1 8 1 90 1 74 1 84 2 12 1 92
P 2 1 2 19 2 06 2 23 2 46 2 25
S 2 5 2 58 2 44 2 65 2 64 2 59
Cl 3 0 3 16 2 83 3 09 3 05 2 87
Ge 1 8 2 01 2 02 1 79 2 14 1 99
As 2 0 2 18 2 20 2 11 2 25 2 21
Se 2 4 2 55 2 48 2 43 2 46 2 42
Br 2 8 2 96 2 74 2 77 2 83 2 69
Sn 1 8 1 96 1 72 1 64 2 12 1 82
I 2 5 2 66 2 21 2 47 2 57 2 36
a. All numerical values are scaled to the original Pauling scale.
b. L. Pauling, The Nature of the Chemical Bond, 3rd Edition, Cornell University Press, Ithaca, NY, 1960.
c. A. L. Allred , J. Inorg. Nucl. Chem., 17, 215 (1961).
d. A. L. Allred and E.G. Rochow, J. Inorg. Nucl. Chem., 5, 264 (1958).
e. Y. R. Luo and S.W. Benson, Acc. Chem. Res., 25, 375 (1992).
f. D. Bergman and J. Hinze, Angew. Chem. Int. Ed. Engl., 35, 150 (1996).
g. L. C. Allen, Int. J. Quantum Chem., 49, 253 (1994).
1.1.4. Electronegativity Equalization
The concept of electronegativity equalization was introduced by
R. T. Sanderson. 13 The idea is implicit in the concept of a molecule as consisting of
nuclei embedded in an electronic cloud and leads to the conclusion that the electron
density will reach an equilibrium state in which there is no net force on the electrons.
The idea of electronegativity equalization finds a theoretical foundation in density
functional theory (see Section 1.3). Several numerical schemes have been developed
for the assignment of charges based on the idea that electronegativity equalization
must be achieved. Sanderson’s initial approach averaged all atoms of a single element,
e.g., carbon, in a molecule and did not distinguish among them. This limitation was
14
addressed by Hercules and co-workers, who assigned electronegativity values called
SR values to specific groups within a molecule. For example, the methyl and ethyl
groups, respectively, were derived from the number of C and H atoms in the group:
3 1/4
SR = SR ×SR (1.6)
CH3 C H
3 1/4 1/4
2
SR = SR ×SR SR ×SR (1.7)
C2H5 C H C H
13 R. T. Sanderson, Chemical Bonds and Bond Energies, Academic Press, New York, 1976;
R. T. Sanderson, Polar Covalence, Academic Press, New York, 1983.
14
J. C. Carver, R. C. Gray, and D. M. Hercules, J. Am. Chem. Soc., 96, 6851 (1974).