Page 36 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 36
well as other hardness/softness relationships are consistent with the idea that hardness 15
and softness are manifestations of the influence of nuclear charge on polarizability.
For polyatomic molecules and ions, hardness and softness are closely related SECTION 1.1
to the HOMO and LUMO energies, which are analogous to the IP and EA values Description of Molecular
Structure Using Valence
for atoms. The larger the HOMO-LUMO gap, the greater the hardness. Numerically, Bond Concepts
hardness is approximately equal to half the energy gap, as defined above for atoms.
In general, chemical reactivity increases as LUMO energies are lower and HOMO
energies are higher. The implication is that softer chemical species, those with smaller
HOMO-LUMO gaps, tend to be more reactive than harder ones. In qualitative terms,
this can be described as the ability of nucleophiles or bases to donate electrons more
readily to electrophiles or acids and begin the process of bond formation. Interactions
between harder chemical entities are more likely to be dominated by electrostatic
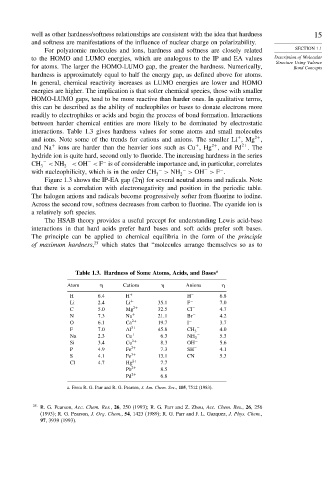
interactions. Table 1.3 gives hardness values for some atoms and small molecules
+
2+
and ions. Note some of the trends for cations and anions. The smaller Li ,Mg ,
2+
+
2+
and Na + ions are harder than the heavier ions such as Cu ,Hg , and Pd . The
hydride ion is quite hard, second only to fluoride. The increasing hardness in the series
−
−
CH 3 − < NH 2 − < OH < F is of considerable importance and, in particular, correlates
−
with nucleophilicity, which is in the order CH 3 − > NH 2 − > OH > F .
−
Figure 1.3 shows the IP-EA gap 2 for several neutral atoms and radicals. Note
that there is a correlation with electronegativity and position in the periodic table.
The halogen anions and radicals become progressively softer from fluorine to iodine.
Across the second row, softness decreases from carbon to fluorine. The cyanide ion is
a relatively soft species.
The HSAB theory provides a useful precept for understanding Lewis acid-base
interactions in that hard acids prefer hard bases and soft acids prefer soft bases.
The principle can be applied to chemical equilibria in the form of the principle
of maximum hardness, 25 which states that “molecules arrange themselves so as to
Table 1.3. Hardness of Some Atoms, Acids, and Bases a
Atom Cations Anions
H 6 4 H + H − 6 8
Li 2 4 Li + 35 1 F − 7 0
C 5 0 Mg 2+ 32 5 Cl − 4 7
N 7 3 Na + 21 1 Br − 4 2
O 6 1 Ca 2+ 19 7 I − 3 7
F 7 0 Al 3+ 45 8 CH 3 − 4 0
Na 2 3 Cu + 6 3 NH 2 − 5 3
Si 3 4 Cu 2+ 8 3 OH − 5 6
P 4 9 Fe 2+ 7 3 SH − 4 1
S 4 1 Fe 3+ 13 1 CN − 5 3
Cl 4 7 Hg 2+ 7 7
Pb 2+ 8 5
Pd 2+ 6 8
a. From R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 105, 7512 (1983).
25
R. G. Pearson, Acc. Chem. Res., 26, 250 (1993); R. G. Parr and Z. Zhou, Acc. Chem. Res., 26, 256
(1993); R. G. Pearson, J. Org. Chem., 54, 1423 (1989); R. G. Parr and J. L. Gazquez, J. Phys. Chem.,
97, 3939 (1993).