Page 31 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 31
10 where IP and IP are the ionization potentials of the s and p electrons and a and b
p
s
are the number of s and p electrons, respectively.
CHAPTER 1
The values on this scale correlate well with the Pauling and Allred-Rochow
Chemical Bonding scales. One feature of this scale is that the IP values can be measured accurately so
and Molecular Structure
that electronegativity becomes an experimentally measured quantity. When the same
concepts are applied to atoms in molecules, the atom undergoes an electronegativity
adjustment that can be related to the energy of its orbitals (as expressed by molecular
orbital theory). The average adjusted energy of an electron is called the energy index
(EI). The EI values of two bound atoms provide a measure of bond polarity called the
bond polarity index 10 (BPI), formulated as
BPI AB = EI −EI A ref − EI −EI B ref (1.5)
B
A
where EI ref are parameters of A−A and B−B bonds.
These approaches, along with several others, give electronegativity scales that are
in good relative agreement in assessing the electron-attracting power of the elements.
Each scale is based on fundamental atomic properties. However, they are in different
units and therefore not directly comparable. Table 1.1 gives the values assigned by
some of the electronegativity scales. The numerical values are scaled to the original
Pauling range. At this point, we wish to emphasize the broad consistency of the values,
not the differences. We use the order of the electronegativity of the elements in a
qualitative way, primarily when discussing bond polarity. It should be noted, however,
that the concept of electronegativity has evolved from an empirical scale to one with
specific physical meaning. We pursue the relationship between these scales further in
Topic 1.5.3.
The most obvious consequence of differential electronegativity is that covalent
bonds between different elements are polar. Each atom bears a partial charge reflecting
the relative electronegativity of the two elements sharing the bond. These charges can
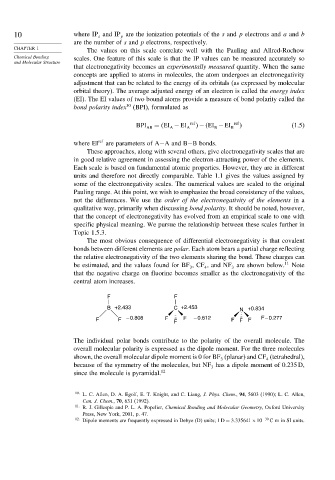
be estimated, and the values found for BF ,CF , and NF are shown below. 11 Note
3
4
3
that the negative charge on fluorine becomes smaller as the electronegativity of the
central atom increases.
F F
B +2.433 C +2.453 N +0.834
F F – 0.808 F F F – 0.612 F F F F – 0.277
The individual polar bonds contribute to the polarity of the overall molecule. The
overall molecular polarity is expressed as the dipole moment. For the three molecules
shown, the overall molecular dipole moment is 0 for BF (planar) and CF (tetrahedral),
3 4
because of the symmetry of the molecules, but NF has a dipole moment of 0.235 D,
3
since the molecule is pyramidal. 12
10
L. C. Allen, D. A. Egolf, E. T. Knight, and C. Liang, J. Phys. Chem., 94, 5603 (1990); L. C. Allen,
Can. J. Chem., 70, 631 (1992).
11 R. J. Gillespie and P. L. A. Popelier, Chemical Bonding and Molecular Geometry, Oxford University
Press, New York, 2001, p. 47.
12 −30
Dipole moments are frequently expressed in Debye (D) units; 1D = 3 335641×10 C m in SI units.