Page 27 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 27
6
CHAPTER 1
Chemical Bonding
and Molecular Structure
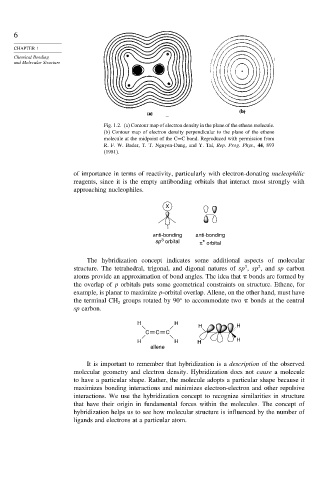
Fig. 1.2. (a) Contour map of electron density in the plane of the ethene molecule.
(b) Contour map of electron density perpendicular to the plane of the ethene
molecule at the midpoint of the C=C bond. Reproduced with permission from
R. F. W. Bader, T. T. Nguyen-Dang, and Y. Tal, Rep. Prog. Phys., 44, 893
(1981).
of importance in terms of reactivity, particularly with electron-donating nucleophilic
reagents, since it is the empty antibonding orbitals that interact most strongly with
approaching nucleophiles.
X
anti-bonding anti-bonding
3
sp orbital π* orbital
The hybridization concept indicates some additional aspects of molecular
2
3
structure. The tetrahedral, trigonal, and digonal natures of sp , sp , and sp carbon
atoms provide an approximation of bond angles. The idea that bonds are formed by
the overlap of p orbitals puts some geometrical constraints on structure. Ethene, for
example, is planar to maximize p-orbital overlap. Allene, on the other hand, must have
the terminal CH groups rotated by 90 to accommodate two bonds at the central
2
sp carbon.
H H
H H
C C C
H H H H
allene
It is important to remember that hybridization is a description of the observed
molecular geometry and electron density. Hybridization does not cause a molecule
to have a particular shape. Rather, the molecule adopts a particular shape because it
maximizes bonding interactions and minimizes electron-electron and other repulsive
interactions. We use the hybridization concept to recognize similarities in structure
that have their origin in fundamental forces within the molecules. The concept of
hybridization helps us to see how molecular structure is influenced by the number of
ligands and electrons at a particular atom.