Page 41 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 41
20 3. The more stable Lewis structures make the largest contribution to the weighted
composite structure. Structures that have the following features are more stable
CHAPTER 1 and make the largest contribution: (a) maximum number of bonds, (b) minimum
Chemical Bonding separation of opposite charges, and (c) charge distribution that is consistent
and Molecular Structure
with relative electronegativity.
Resonance structures are used to convey the structural and electron distribution conse-
quences of conjugation and delocalization. Let us look specifically at 1,3-butadiene,
1,3,5-hexatriene, prop-2-enal (acrolein), methoxyethene (methyl vinyl ether), and
ethenamine (vinylamine) to illustrate how resonance can help us understand electron
distribution and reactivity. The hybridization picture for 1,3-butadiene suggests that
there can be overlap of the p orbitals. Thermodynamic analysis (see Section 3.1.2.3)
indicates that there is a net stabilization of about 3–4 kcal/mol, relative to two isolated
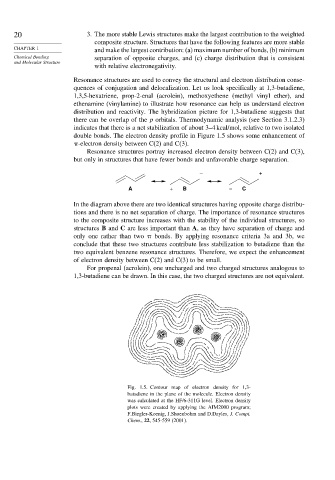
double bonds. The electron density profile in Figure 1.5 shows some enhancement of
-electron density between C(2) and C(3).
Resonance structures portray increased electron density between C(2) and C(3),
but only in structures that have fewer bonds and unfavorable charge separation.
– +
A + B – C
In the diagram above there are two identical structures having opposite charge distribu-
tions and there is no net separation of charge. The importance of resonance structures
to the composite structure increases with the stability of the individual structures, so
structures B and C are less important than A, as they have separation of charge and
only one rather than two bonds. By applying resonance criteria 3a and 3b, we
conclude that these two structures contribute less stabilization to butadiene than the
two equivalent benzene resonance structures. Therefore, we expect the enhancement
of electron density between C(2) and C(3) to be small.
For propenal (acrolein), one uncharged and two charged structures analogous to
1,3-butadiene can be drawn. In this case, the two charged structures are not equivalent.
Fig. 1.5. Contour map of electron density for 1,3-
butadiene in the plane of the molecule. Electron density
was calculated at the HF/6-311G level. Electron density
plots were created by applying the AIM2000 program;
F.Biegler-Koenig, J.Shoenbohm and D.Dayles, J. Compt.
Chem., 22, 545-559 (2001).