Page 46 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 46
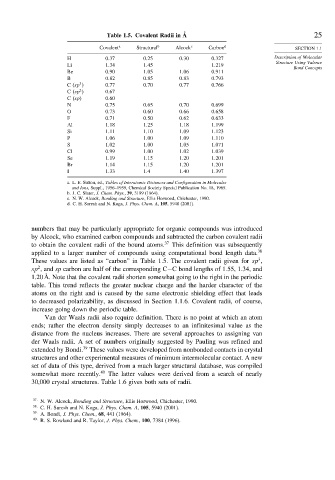
Table 1.5. Covalent Radii in Å 25
Covalent a Structural b Alcock c Carbon d SECTION 1.1
H 0 37 0 25 0 30 0 327 Description of Molecular
Structure Using Valence
Li 1 34 1 45 1 219
Bond Concepts
Be 0 90 1 05 1 06 0 911
B 0 82 0 85 0 83 0 793
3
C sp 0 77 0 70 0 77 0 766
2
C sp 0 67
C sp 0 60
N 0 75 0 65 0 70 0 699
O 0 73 0 60 0 66 0 658
F 0 71 0 50 0 62 0 633
Al 1 18 1 25 1 18 1 199
Si 1 11 1 10 1 09 1 123
P 1 06 1 00 1 09 1 110
S 1 02 1 00 1 05 1 071
Cl 0 99 1 00 1 02 1 039
Se 1 19 1 15 1 20 1 201
Br 1 14 1 15 1 20 1 201
I 1 33 1 4 1 40 1 397
a. L. E. Sutton, ed., Tables of Interatomic Distances and Configuration in Molecules
and Ions, Suppl., 1956–1959, Chemical Society Special Publication No. 18, 1965.
b. J. C. Slater, J. Chem. Phys., 39, 3199 (1964).
c. N. W. Alcock, Bonding and Structure, Ellis Horwood, Chichester, 1990.
d. C. H. Suresh and N. Koga, J. Phys. Chem. A, 105, 5940 (2001).
numbers that may be particularly appropriate for organic compounds was introduced
by Alcock, who examined carbon compounds and subtracted the carbon covalent radii
to obtain the covalent radii of the bound atoms. 37 This definition was subsequently
applied to a larger number of compounds using computational bond length data. 38
3
These values are listed as “carbon” in Table 1.5. The covalent radii given for sp ,
2
sp , and sp carbon are half of the corresponding C−C bond lengths of 1.55, 1.34, and
1.20 Å. Note that the covalent radii shorten somewhat going to the right in the periodic
table. This trend reflects the greater nuclear charge and the harder character of the
atoms on the right and is caused by the same electronic shielding effect that leads
to decreased polarizability, as discussed in Section 1.1.6. Covalent radii, of course,
increase going down the periodic table.
Van der Waals radii also require definition. There is no point at which an atom
ends; rather the electron density simply decreases to an infinitesimal value as the
distance from the nucleus increases. There are several approaches to assigning van
der Waals radii. A set of numbers originally suggested by Pauling was refined and
39
extended by Bondi. These values were developed from nonbonded contacts in crystal
structures and other experimental measures of minimum intermolecular contact. A new
set of data of this type, derived from a much larger structural database, was compiled
somewhat more recently. 40 The latter values were derived from a search of nearly
30,000 crystal structures. Table 1.6 gives both sets of radii.
37
N. W. Alcock, Bonding and Structure, Ellis Horwood, Chichester, 1990.
38
C. H. Suresh and N. Koga, J. Phys. Chem. A, 105, 5940 (2001).
39 A. Bondi, J. Phys. Chem., 68, 441 (1964).
40
R. S. Rowland and R. Taylor, J. Phys. Chem., 100, 7384 (1996).