Page 51 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 51
30
CHAPTER 1
Chemical Bonding
and Molecular Structure
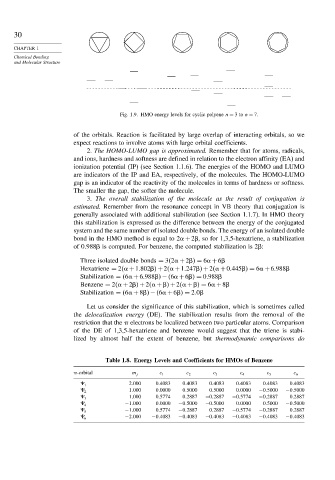
Fig. 1.9. HMO energy levels for cyclic polyene n = 3to n = 7.
of the orbitals. Reaction is facilitated by large overlap of interacting orbitals, so we
expect reactions to involve atoms with large orbital coefficients.
2. The HOMO-LUMO gap is approximated. Remember that for atoms, radicals,
and ions, hardness and softness are defined in relation to the electron affinity (EA) and
ionization potential (IP) (see Section 1.1.6). The energies of the HOMO and LUMO
are indicators of the IP and EA, respectively, of the molecules. The HOMO-LUMO
gap is an indicator of the reactivity of the molecules in terms of hardness or softness.
The smaller the gap, the softer the molecule.
3. The overall stabilization of the molecule as the result of conjugation is
estimated. Remember from the resonance concept in VB theory that conjugation is
generally associated with additional stabilization (see Section 1.1.7). In HMO theory
this stabilization is expressed as the difference between the energy of the conjugated
system and the same number of isolated double bonds. The energy of an isolated double
bond in the HMO method is equal to 2 +2 , so for 1,3,5-hexatriene, a stabilization
of 0 988 is computed. For benzene, the computed stabilization is 2 :
Three isolated double bonds = 3 2 +2 = 6 +6
Hexatriene = 2 +1 802 +2 +1 247 +2 +0 445 = 6 +6 988
Stabilization = 6 +6 988 − 6 +6 = 0 988
Benzene = 2 +2 +2 + +2 + = 6 +8
Stabilization = 6 +8 − 6 +6 = 2 0
Let us consider the significance of this stabilization, which is sometimes called
the delocalization energy (DE). The stabilization results from the removal of the
restriction that the electrons be localized between two particular atoms. Comparison
of the DE of 1,3,5-hexatriene and benzene would suggest that the triene is stabi-
lized by almost half the extent of benzene, but thermodynamic comparisons do
Table 1.8. Energy Levels and Coefficients for HMOs of Benzene
-orbital m j c 1 c 2 c 3 c 4 c 5 c 6
2 000 0 4083 0 4083 0 4083 0 4083 0 4083 0 4083
1
1 000 0 0000 0 5000 0 5000 0 0000 −0 5000 −0 5000
2
1 000 0 5774 0 2887 −0 2887 −0 5774 −0 2887 0 2887
3
−1 000 0 0000 −0 5000 −0 5000 0 0000 0 5000 −0 5000
4
−1 000 0 5774 −0 2887 0 2887 −0 5774 −0 2887 0 2887
5
−2 000 −0 4083 −0 4083 −0 4083 −0 4083 −0 4083 −0 4083
6