Page 52 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 52
31
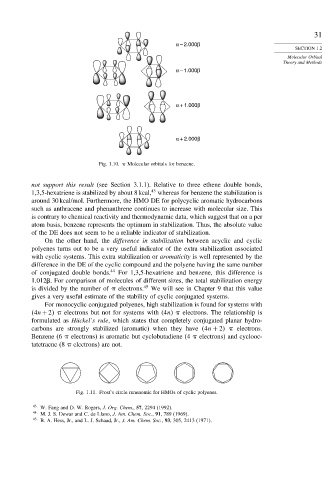
α – 2.000β
SECTION 1.2
Molecular Orbital
Theory and Methods
α – 1.000β
α + 1.000β
α + 2.000β
Fig. 1.10. Molecular orbitals for benzene.
not support this result (see Section 3.1.1). Relative to three ethene double bonds,
43
1,3,5-hexatriene is stabilized by about 8 kcal, whereas for benzene the stabilization is
around 30 kcal/mol. Furthermore, the HMO DE for polycyclic aromatic hydrocarbons
such as anthracene and phenanthrene continues to increase with molecular size. This
is contrary to chemical reactivity and thermodynamic data, which suggest that on a per
atom basis, benzene represents the optimum in stabilization. Thus, the absolute value
of the DE does not seem to be a reliable indicator of stabilization.
On the other hand, the difference in stabilization between acyclic and cyclic
polyenes turns out to be a very useful indicator of the extra stabilization associated
with cyclic systems. This extra stabilization or aromaticity is well represented by the
difference in the DE of the cyclic compound and the polyene having the same number
of conjugated double bonds. 44 For 1,3,5-hexatriene and benzene, this difference is
1 012 . For comparison of molecules of different sizes, the total stabilization energy
is divided by the number of electrons. 45 We will see in Chapter 9 that this value
gives a very useful estimate of the stability of cyclic conjugated systems.
For monocyclic conjugated polyenes, high stabilization is found for systems with
4n + 2 electrons but not for systems with (4n) electrons. The relationship is
formulated as Hückel’s rule, which states that completely conjugated planar hydro-
carbons are strongly stabilized (aromatic) when they have 4n + 2 electrons.
Benzene (6 electrons) is aromatic but cyclobutadiene (4 electrons) and cyclooc-
tatetraene (8 electrons) are not.
Fig. 1.11. Frost’s circle mnenomic for HMOs of cyclic polyenes.
43 W. Fang and D. W. Rogers, J. Org. Chem., 57, 2294 (1992).
44 M. J. S. Dewar and C. de Llano, J. Am. Chem. Soc., 91, 789 (1969).
45
B. A. Hess, Jr., and L. J. Schaad, Jr., J. Am. Chem. Soc., 93, 305, 2413 (1971).