Page 54 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 54
Specific ab initio methods are characterized by the form of the wave function 33
and the nature of the basis set functions that are used. The most common form of
the wave function is the single determinant of molecular orbitals expressed as a linear SECTION 1.2
combination of basis functions, as is the case with semiempirical calculations. We Molecular Orbital
Theory and Methods
describe alternatives later in this section. Early calculations were often done with Slater
functions, designated STO for Slater-type orbitals. Currently most computations are
done with Gaussian basis functions, designated by GTOs. A fairly accurate represen-
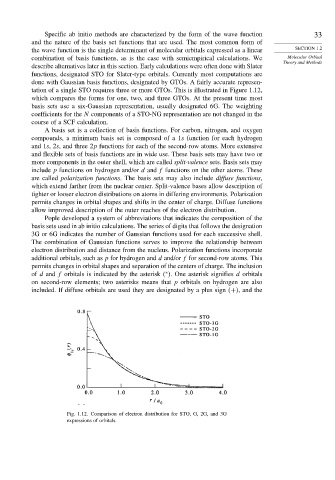
tation of a single STO requires three or more GTOs. This is illustrated in Figure 1.12,
which compares the forms for one, two, and three GTOs. At the present time most
basis sets use a six-Gaussian representation, usually designated 6G. The weighting
coefficients for the N components of a STO-NG representation are not changed in the
course of a SCF calculation.
A basis set is a collection of basis functions. For carbon, nitrogen, and oxygen
compounds, a minimum basis set is composed of a 1s function for each hydrogen
and 1s,2s, and three 2p functions for each of the second-row atoms. More extensive
and flexible sets of basis functions are in wide use. These basis sets may have two or
more components in the outer shell, which are called split-valence sets. Basis sets may
include p functions on hydrogen and/or d and f functions on the other atoms. These
are called polarization functions. The basis sets may also include diffuse functions,
which extend farther from the nuclear center. Split-valence bases allow description of
tighter or looser electron distributions on atoms in differing environments. Polarization
permits changes in orbital shapes and shifts in the center of charge. Diffuse functions
allow improved description of the outer reaches of the electron distribution.
Pople developed a system of abbreviations that indicates the composition of the
basis sets used in ab initio calculations. The series of digits that follows the designation
3G or 6G indicates the number of Gaussian functions used for each successive shell.
The combination of Gaussian functions serves to improve the relationship between
electron distribution and distance from the nucleus. Polarization functions incorporate
additional orbitals, such as p for hydrogen and d and/or f for second-row atoms. This
permits changes in orbital shapes and separation of the centers of charge. The inclusion
of d and f orbitals is indicated by the asterisk . One asterisk signifies d orbitals
∗
on second-row elements; two asterisks means that p orbitals on hydrogen are also
included. If diffuse orbitals are used they are designated by a plus sign + , and the
Fig. 1.12. Comparison of electron distribution for STO, G, 2G, and 3G
expressions of orbitals.