Page 58 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 58
37
SECTION 1.2
A Molecular Orbital
Theory and Methods
S
A
S
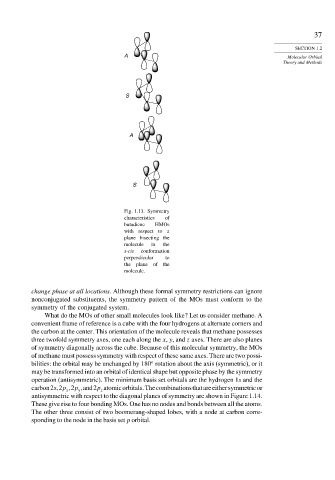
Fig. 1.13. Symmetry
characteristics of
butadiene HMOs
with respect to a
plane bisecting the
molecule in the
s-cis conformation
perpendicular to
the plane of the
molecule.
change phase at all locations. Although these formal symmetry restrictions can ignore
nonconjugated substituents, the symmetry pattern of the MOs must conform to the
symmetry of the conjugated system.
What do the MOs of other small molecules look like? Let us consider methane. A
convenient frame of reference is a cube with the four hydrogens at alternate corners and
the carbon at the center. This orientation of the molecule reveals that methane possesses
three twofold symmetry axes, one each along the x, y, and z axes. There are also planes
of symmetry diagonally across the cube. Because of this molecular symmetry, the MOs
of methane must possess symmetry with respect of these same axes. There are two possi-
bilities: the orbital may be unchanged by 180 rotation about the axis (symmetric), or it
may be transformed into an orbital of identical shape but opposite phase by the symmetry
operation (antisymmetric). The minimum basis set orbitals are the hydrogen 1s and the
carbon2s,2p ,2p ,and2p atomicorbitals.Thecombinationsthatareeithersymmetricor
x
y
z
antisymmetric with respect to the diagonal planes of symmetry are shown in Figure 1.14.
These give rise to four bonding MOs. One has no nodes and bonds between all the atoms.
The other three consist of two boomerang-shaped lobes, with a node at carbon corre-
sponding to the node in the basis set p orbital.