Page 60 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 60
39
SECTION 1.2
Molecular Orbital
Theory and Methods
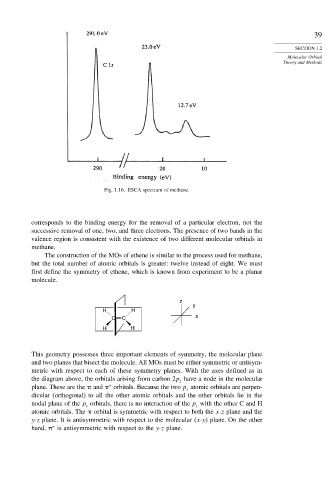
Fig. 1.16. ESCA spectrum of methane.
corresponds to the binding energy for the removal of a particular electron, not the
successive removal of one, two, and three electrons. The presence of two bands in the
valence region is consistent with the existence of two different molecular orbitals in
methane.
The construction of the MOs of ethene is similar to the process used for methane,
but the total number of atomic orbitals is greater: twelve instead of eight. We must
first define the symmetry of ethene, which is known from experiment to be a planar
molecule.
z
y
H H
C C x
H H
This geometry possesses three important elements of symmetry, the molecular plane
and two planes that bisect the molecule. All MOs must be either symmetric or antisym-
metric with respect to each of these symmetry planes. With the axes defined as in
the diagram above, the orbitals arising from carbon 2p have a node in the molecular
z
∗
plane. These are the and orbitals. Because the two p atomic orbitals are perpen-
z
dicular (orthogonal) to all the other atomic orbitals and the other orbitals lie in the
nodal plane of the p orbitals, there is no interaction of the p with the other C and H
z
z
atomic orbitals. The orbital is symmetric with respect to both the x-z plane and the
y-z plane. It is antisymmetric with respect to the molecular x-y plane. On the other
hand, is antisymmetric with respect to the y-z plane.
∗