Page 63 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 63
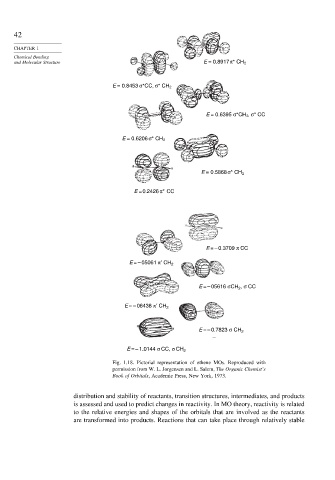
42
CHAPTER 1
Chemical Bonding
and Molecular Structure E = 0.8917 π* CH 2
E = 0.8453 σ*CC, σ* CH 2
E = 0.6395 σ*CH 2 , σ* CC
E = 0.6206 σ* CH 2
E = 0.5868 σ* CH 2
E = 0.2426 π*CC
E = – 0.3709 π CC
E = – 05061 π′ CH 2
E = – 05616 σCH , σ CC
2
E = – 06438 π′ CH 2
E = – 0.7823 σ CH 2
E = – 1.0144 σ CC, σ CH 2
Fig. 1.18. Pictorial representation of ethene MOs. Reproduced with
permission from W. L. Jorgensen and L. Salem, The Organic Chemist’s
Book of Orbitals, Academic Press, New York, 1973.
distribution and stability of reactants, transition structures, intermediates, and products
is assessed and used to predict changes in reactivity. In MO theory, reactivity is related
to the relative energies and shapes of the orbitals that are involved as the reactants
are transformed into products. Reactions that can take place through relatively stable