Page 66 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 66
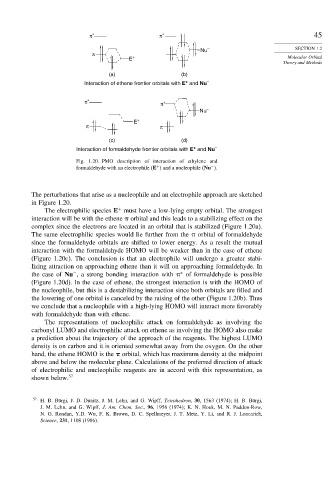
π ∗ π ∗ 45
Nu – SECTION 1.2
π
E + Molecular Orbital
Theory and Methods
(a) (b)
+
Interaction of ethene frontier orbitals with E and Nu –
π ∗ π ∗
Nu –
E +
π π
(c) (d)
+
Interaction of formaldehyde frontier orbitals with E and Nu –
Fig. 1.20. PMO description of interaction of ethylene and
−
+
formaldehyde with an electrophile E and a nucleophile Nu .
The perturbations that arise as a nucleophile and an electrophile approach are sketched
in Figure 1.20.
The electrophilic species E must have a low-lying empty orbital. The strongest
+
interaction will be with the ethene orbital and this leads to a stabilizing effect on the
complex since the electrons are located in an orbital that is stabilized (Figure 1.20a).
The same electrophilic species would lie further from the orbital of formaldehyde
since the formaldehyde orbitals are shifted to lower energy. As a result the mutual
interaction with the formaldehyde HOMO will be weaker than in the case of ethene
(Figure 1.20c). The conclusion is that an electrophile will undergo a greater stabi-
lizing attraction on approaching ethene than it will on approaching formaldehyde. In
−
∗
the case of Nu , a strong bonding interaction with of formaldehyde is possible
(Figure 1.20d). In the case of ethene, the strongest interaction is with the HOMO of
the nucleophile, but this is a destabilizing interaction since both orbitals are filled and
the lowering of one orbital is canceled by the raising of the other (Figure 1.20b). Thus
we conclude that a nucleophile with a high-lying HOMO will interact more favorably
with formaldehyde than with ethene.
The representations of nucleophilic attack on formaldehyde as involving the
carbonyl LUMO and electrophilic attack on ethene as involving the HOMO also make
a prediction about the trajectory of the approach of the reagents. The highest LUMO
density is on carbon and it is oriented somewhat away from the oxygen. On the other
hand, the ethene HOMO is the orbital, which has maximum density at the midpoint
above and below the molecular plane. Calculations of the preferred direction of attack
of electrophilic and nucleophilic reagents are in accord with this representation, as
shown below. 57
57
H. B. Bürgi, J. D. Dunitz, J. M. Lehn, and G. Wipff, Tetrahedron, 30, 1563 (1974); H. B. Bürgi,
J. M. Lehn, and G. Wipff, J. Am. Chem. Soc., 96, 1956 (1974); K. N. Houk, M. N. Paddon-Row,
N. G. Rondan, Y.D. Wu, F. K. Brown, D. C. Spellmeyer, J. T. Metz, Y. Li, and R. J. Loncarich,
Science, 231, 1108 (1986).