Page 70 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 70
49
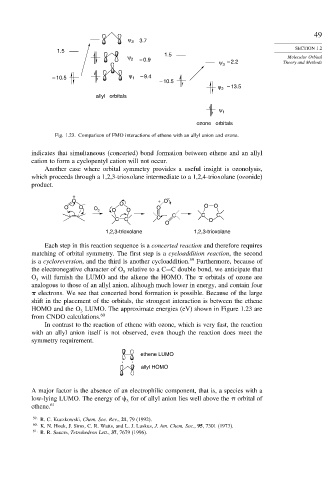
ψ 3 3.7
SECTION 1.2
1.5
ψ 2 – 0.9 1.5 Molecular Orbital
ψ – 2.2 Theory and Methods
3
– 10.5 ψ 1 – 9.4
– 10.5
ψ 2 – 13.5
allyl orbitals
ψ 1
ozone orbitals
Fig. 1.23. Comparison of FMO interactions of ethene with an allyl anion and ozone.
indicates that simultaneous (concerted) bond formation between ethene and an allyl
cation to form a cyclopentyl cation will not occur.
Another case where orbital symmetry provides a useful insight is ozonolysis,
which proceeds through a 1,2,3-trioxolane intermediate to a 1,2,4-trioxolane (ozonide)
product.
+ _
O _ O + O
O O O O O O
3 O O C C
C C C C O
O
1,2,3-trioxolane 1,2,3-trioxolane
Each step in this reaction sequence is a concerted reaction and therefore requires
matching of orbital symmetry. The first step is a cycloaddition reaction, the second
is a cycloreversion, and the third is another cycloaddition. 59 Furthermore, because of
the electronegative character of O relative to a C=C double bond, we anticipate that
3
O will furnish the LUMO and the alkene the HOMO. The orbitals of ozone are
3
analogous to those of an allyl anion, although much lower in energy, and contain four
electrons. We see that concerted bond formation is possible. Because of the large
shift in the placement of the orbitals, the strongest interaction is between the ethene
HOMO and the O LUMO. The approximate energies (eV) shown in Figure 1.23 are
3
from CNDO calculations. 60
In contrast to the reaction of ethene with ozone, which is very fast, the reaction
with an allyl anion itself is not observed, even though the reaction does meet the
symmetry requirement.
ethene LUMO
allyl HOMO
A major factor is the absence of an electrophilic component, that is, a species with a
low-lying LUMO. The energy of for of allyl anion lies well above the orbital of
3
ethene. 61
59 R. C. Kuczkowski, Chem. Soc. Rev., 21, 79 (1992).
60 K. N. Houk, J. Sims, C. R. Watts, and L. J. Luskus, J. Am. Chem. Soc., 95, 7301 (1973).
61
R. R. Sauers, Tetrahedron Lett., 37, 7679 (1996).