Page 72 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 72
can adjust the relative energy of molecules. Species with substantial charge separation 51
will be stabilized most strongly.
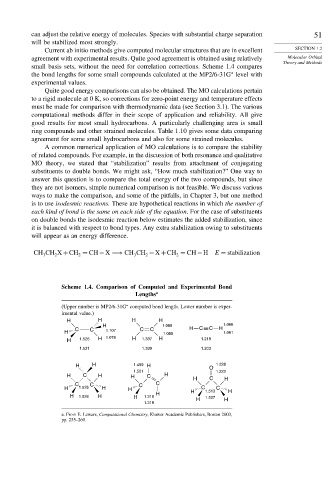
Current ab initio methods give computed molecular structures that are in excellent SECTION 1.2
agreement with experimental results. Quite good agreement is obtained using relatively Molecular Orbital
Theory and Methods
small basis sets, without the need for correlation corrections. Scheme 1.4 compares
∗
the bond lengths for some small compounds calculated at the MP2/6-31G level with
experimental values.
Quite good energy comparisons can also be obtained. The MO calculations pertain
to a rigid molecule at 0 K, so corrections for zero-point energy and temperature effects
must be made for comparison with thermodynamic data (see Section 3.1). The various
computational methods differ in their scope of application and reliability. All give
good results for most small hydrocarbons. A particularly challenging area is small
ring compounds and other strained molecules. Table 1.10 gives some data comparing
agreement for some small hydrocarbons and also for some strained molecules.
A common numerical application of MO calculations is to compare the stability
of related compounds. For example, in the discussion of both resonance and qualitative
MO theory, we stated that “stabilization” results from attachment of conjugating
substituents to double bonds. We might ask, “How much stabilization?” One way to
answer this question is to compare the total energy of the two compounds, but since
they are not isomers, simple numerical comparison is not feasible. We discuss various
ways to make the comparison, and some of the pitfalls, in Chapter 3, but one method
is to use isodesmic reactions. These are hypothetical reactions in which the number of
each kind of bond is the same on each side of the equation. For the case of substituents
on double bonds the isodesmic reaction below estimates the added stabilization, since
it is balanced with respect to bond types. Any extra stabilization owing to substituents
will appear as an energy difference.
CH CH X +CH = CH−X −→ CH CH −X +CH = CH−H E = stabilization
3
2
2
2
2
3
Scheme 1.4. Comparison of Computed and Experimental Bond
Lengths a
(Upper number is MP2/6-31G computed bond length. Lower number is exper-
∗
imental value.)
H H H H
H 1.085 1.066
C C 1.107 C C H C C H
H 1.085 1.061
H 1.526 H 1.078 H 1.337 H 1.218
1.531 1.339 1.203
H H 1.499 H O 1.228
1.501 1.222
H C H H C H
H C H
C C C C
H 1.526 H H C 1.513 C H
H H
H 1.526 H H 1.318 H 1.507 H
1.318
a. From E. Lewars, Computational Chemistry, Kluwer Academic Publishers, Boston 2003,
pp. 255–260.