Page 75 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 75
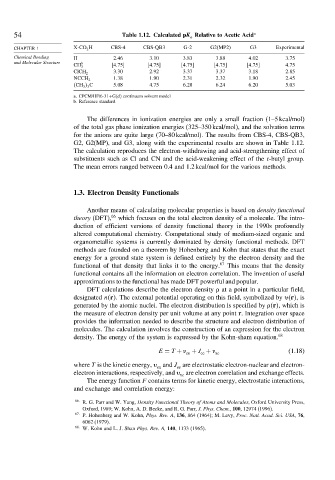
54 Table 1.12. Calculated pK Relative to Acetic Acid a
a
CHAPTER 1 X-CO 2 H CBS-4 CBS-QB3 G-2 G2(MP2) G3 Experimental
Chemical Bonding H 2 46 3 10 3 83 3 88 4 02 3 75
and Molecular Structure b
CH 3 4 75 4 75 4 75 4 75 4 75 4 75
3 30 2 92 3 37 3 37 3 18 2 85
ClCH 2
1 38 1 90 2 31 2 32 1 90 2 45
NCCH 2
CH 3 3 C 5 08 4 75 6 28 6 24 6 20 5 03
a. CPCM/HF/6-31+G(d) continuum solvent model
b. Reference standard.
The differences in ionization energies are only a small fraction (1–5 kcal/mol)
of the total gas phase ionization energies (325–350 kcal/mol), and the solvation terms
for the anions are quite large (70–80 kcal/mol). The results from CBS-4, CBS-QB3,
G2, G2(MP), and G3, along with the experimental results are shown in Table 1.12.
The calculation reproduces the electron-withdrawing and acid-strengthening effect of
substituents such as Cl and CN and the acid-weakening effect of the t-butyl group.
The mean errors ranged between 0.4 and 1.2 kcal/mol for the various methods.
1.3. Electron Density Functionals
Another means of calculating molecular properties is based on density functional
theory (DFT), 66 which focuses on the total electron density of a molecule. The intro-
duction of efficient versions of density functional theory in the 1990s profoundly
altered computational chemistry. Computational study of medium-sized organic and
organometallic systems is currently dominated by density functional methods. DFT
methods are founded on a theorem by Hohenberg and Kohn that states that the exact
energy for a ground state system is defined entirely by the electron density and the
functional of that density that links it to the energy. 67 This means that the density
functional contains all the information on electron correlation. The invention of useful
approximations to the functional has made DFT powerful and popular.
DFT calculations describe the electron density at a point in a particular field,
designated n r . The external potential operating on this field, symbolized by r ,is
generated by the atomic nuclei. The electron distribution is specified by r , which is
the measure of electron density per unit volume at any point r. Integration over space
provides the information needed to describe the structure and electron distribution of
molecules. The calculation involves the construction of an expression for the electron
density. The energy of the system is expressed by the Kohn-sham equation. 68
E = T + +J + xc (1.18)
en
ee
where T is the kinetic energy, and J are electrostatic electron-nuclear and electron-
en ee
electron interactions, respectively, and are electron correlation and exchange effects.
xc
The energy function F contains terms for kinetic energy, electrostatic interactions,
and exchange and correlation energy:
66 R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press,
Oxford, 1989; W. Kohn, A. D. Becke, and R. G. Parr, J. Phys. Chem., 100, 12974 (1996).
67 P. Hohenberg and W. Kohn, Phys. Rev. A, 136, 864 (1964); M. Levy, Proc. Natl. Acad. Sci. USA, 76,
6062 (1979).
68
W. Kohn and L. J. Shan Phys. Rev. A, 140, 1133 (1965).