Page 71 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 71
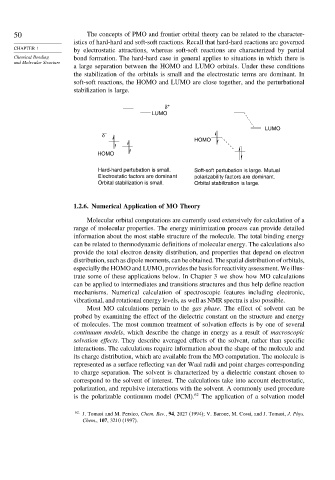
50 The concepts of PMO and frontier orbital theory can be related to the character-
istics of hard-hard and soft-soft reactions. Recall that hard-hard reactions are governed
CHAPTER 1 by electrostatic attractions, whereas soft-soft reactions are characterized by partial
Chemical Bonding bond formation. The hard-hard case in general applies to situations in which there is
and Molecular Structure
a large separation between the HOMO and LUMO orbitals. Under these conditions
the stabilization of the orbitals is small and the electrostatic terms are dominant. In
soft-soft reactions, the HOMO and LUMO are close together, and the perturbational
stabilization is large.
δ +
LUMO
LUMO
δ –
HOMO
HOMO
Hard-hard pertubation is small. Soft-soft pertubation is large. Mutual
Electrostatic factors are dominant polarizability factors are dominant.
Orbital stabilization is small. Orbital stabilization is large.
1.2.6. Numerical Application of MO Theory
Molecular orbital computations are currently used extensively for calculation of a
range of molecular properties. The energy minimization process can provide detailed
information about the most stable structure of the molecule. The total binding energy
can be related to thermodynamic definitions of molecular energy. The calculations also
provide the total electron density distribution, and properties that depend on electron
distribution, such as dipole moments, can be obtained. The spatial distribution of orbitals,
especially the HOMO and LUMO, provides the basis for reactivity assessment. We illus-
trate some of these applications below. In Chapter 3 we show how MO calculations
can be applied to intermediates and transitions structures and thus help define reaction
mechanisms. Numerical calculation of spectroscopic features including electronic,
vibrational, and rotational energy levels, as well as NMR spectra is also possible.
Most MO calculations pertain to the gas phase. The effect of solvent can be
probed by examining the effect of the dielectric constant on the structure and energy
of molecules. The most common treatment of solvation effects is by one of several
continuum models, which describe the change in energy as a result of macroscopic
solvation effects. They describe averaged effects of the solvent, rather than specific
interactions. The calculations require information about the shape of the molecule and
its charge distribution, which are available from the MO computation. The molecule is
represented as a surface reflecting van der Waal radii and point charges corresponding
to charge separation. The solvent is characterized by a dielectric constant chosen to
correspond to the solvent of interest. The calculations take into account electrostatic,
polarization, and repulsive interactions with the solvent. A commonly used procedure
is the polarizable continuum model (PCM). 62 The application of a solvation model
62
J. Tomasi and M. Persico, Chem. Rev., 94, 2027 (1994); V. Barone, M. Cossi, and J. Tomasi, J. Phys.
Chem., 107, 3210 (1997).