Page 67 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 67
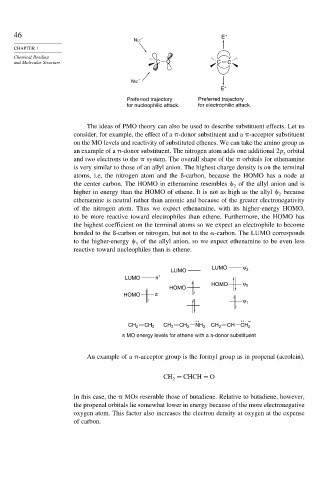
46 E +
Nu: –
CHAPTER 1
Chemical Bonding
and Molecular Structure C O C C
Nu: –
E +
Preferred trajectory Preferred trajectory
for nucleophilic attack. for electrophilic attack.
The ideas of PMO theory can also be used to describe substituent effects. Let us
consider, for example, the effect of a -donor substituent and a -acceptor substituent
on the MO levels and reactivity of substituted ethenes. We can take the amino group as
an example of a -donor substituent. The nitrogen atom adds one additional 2p orbital
z
and two electrons to the system. The overall shape of the orbitals for ethenamine
is very similar to those of an allyl anion. The highest charge density is on the terminal
atoms, i.e, the nitrogen atom and the ß-carbon, because the HOMO has a node at
the center carbon. The HOMO in ethenamine resembles of the allyl anion and is
2
higher in energy than the HOMO of ethene. It is not as high as the allyl because
2
ethenamine is neutral rather than anionic and because of the greater electronegativity
of the nitrogen atom. Thus we expect ethenamine, with its higher-energy HOMO,
to be more reactive toward electrophiles than ethene. Furthermore, the HOMO has
the highest coefficient on the terminal atoms so we expect an electrophile to become
bonded to the ß-carbon or nitrogen, but not to the -carbon. The LUMO corresponds
to the higher-energy of the allyl anion, so we expect ethenamine to be even less
3
reactive toward nucleophiles than is ethene.
LUMO ψ
LUMO 3
LUMO π ∗
HOMO ψ 2
HOMO
HOMO π
ψ 1
–
CH 2 CH 2 CH 2 CH 2 NH 2 CH 2 CH CH 2
π MO energy levels for ethene with a π-donor substituent
An example of a -acceptor group is the formyl group as in propenal (acrolein).
CH = CHCH = O
2
In this case, the MOs resemble those of butadiene. Relative to butadiene, however,
the propenal orbitals lie somewhat lower in energy because of the more electronegative
oxygen atom. This factor also increases the electron density at oxygen at the expense
of carbon.