Page 69 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 69
48 CH 2 CHNH 2 + H + CH CH NH 2 +
3
CHAPTER 1
Nu CH CH CH O –
Chemical Bonding 2
and Molecular Structure H +
Nu: – CH 2 CHCH O NuCH 2 CH 2 CH O
–
Nu CH C HCH O
2
Frontier orbital theory also provides the framework for analysis of the effect that
the orbital symmetry has on reactivity. One of the basic tenets of PMO theory is that
the symmetries of two orbitals must match to permit a strong interaction between
them. This symmetry requirement, used in the context of frontier orbital theory, can
be a very powerful tool for predicting reactivity. As an example, let us examine the
approach of an allyl cation and an ethene molecule and ask whether the following
reaction is likely to occur:
H
H
C ? +
+
H C CH 2
2
H C CH 2
2
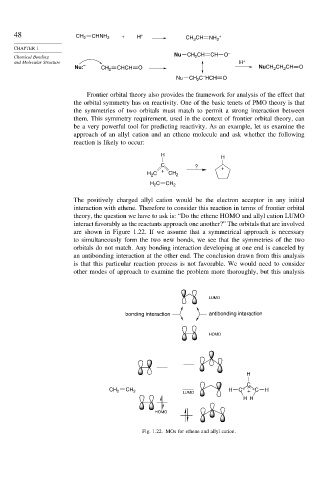
The positively charged allyl cation would be the electron acceptor in any initial
interaction with ethene. Therefore to consider this reaction in terms of frontier orbital
theory, the question we have to ask is: “Do the ethene HOMO and allyl cation LUMO
interact favorably as the reactants approach one another?” The orbitals that are involved
are shown in Figure 1.22. If we assume that a symmetrical approach is necessary
to simultaneously form the two new bonds, we see that the symmetries of the two
orbitals do not match. Any bonding interaction developing at one end is canceled by
an antibonding interaction at the other end. The conclusion drawn from this analysis
is that this particular reaction process is not favorable. We would need to consider
other modes of approach to examine the problem more thoroughly, but this analysis
LUMO
bonding interaction antibonding interaction
HOMO
H
C
CH H C H
CH 2 2 LUMO + C
H H
HOMO
Fig. 1.22. MOs for ethene and allyl cation.