Page 74 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 74
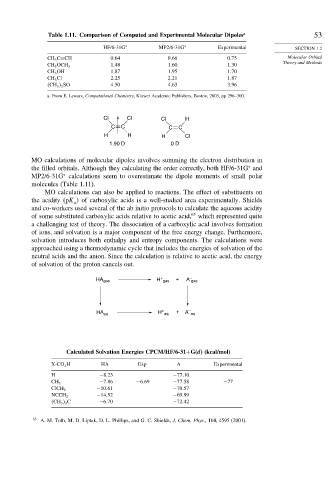
Table 1.11. Comparison of Computed and Experimental Molecular Dipoles a 53
HF/6-31G ∗ MP2/6-31G ∗ Experimental SECTION 1.2
CH 3 C≡CH 0 64 0 66 0 75 Molecular Orbital
Theory and Methods
1 48 1 60 1 30
CH 3 OCH 3
CH 3 OH 1 87 1 95 1 70
CH 3 Cl 2 25 2 21 1 87
CH 3 2 SO 4 50 4 63 3 96
a. From E. Lewars, Computational Chemistry, Kluwer Academic Publishers, Boston, 2003, pp 296–300.
Cl Cl Cl H
C C C C
H H H Cl
1.90 D 0 D
MO calculations of molecular dipoles involves summing the electron distribution in
the filled orbitals. Although they calculating the order correctly, both HF/6-31G and
∗
MP2/6-31G calculations seem to overestimate the dipole moments of small polar
∗
molecules (Table 1.11).
MO calculations can also be applied to reactions. The effect of substituents on
the acidity pK of carboxylic acids is a well-studied area experimentally. Shields
a
and co-workers used several of the ab initio protocols to calculate the aqueous acidity
of some substituted carboxylic acids relative to acetic acid, 65 which represented quite
a challenging test of theory. The dissociation of a carboxylic acid involves formation
of ions, and solvation is a major component of the free energy change. Furthermore,
solvation introduces both enthalpy and entropy components. The calculations were
approached using a thermodynamic cycle that includes the energies of solvation of the
neutral acids and the anion. Since the calculation is relative to acetic acid, the energy
of solvation of the proton cancels out.
+ + –
HA gas H gas A gas
+
–
HA aq H aq + A aq
Calculated Solvation Energies CPCM/HF/6-31+G(d) (kcal/mol)
X-CO 2 H HA Exp A − Experimental
H −8 23 −77 10
−7 86 −6 69 −77 58 −77
CH 3
−10 61 −70 57
ClCH 2
−14 52 −69 99
NCCH 2
CH 3 3 C −6 70 −72 42
65
A. M. Toth, M. D. Liptak, D. L. Phillips, and G. C. Shields, J. Chem. Phys., 114, 4595 (2001).