Page 73 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 73
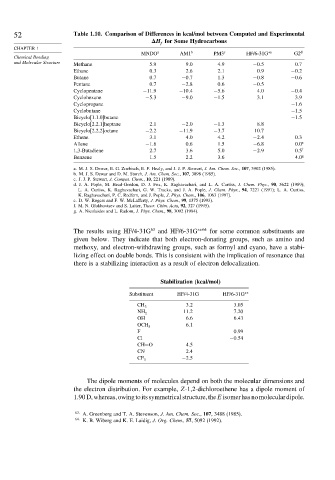
52 Table 1.10. Comparison of Differences in kcal/mol between Computed and Experimental
H for Some Hydrocarbons
f
CHAPTER 1
MNDO a AM1 b PM3 c HF/6-31G ∗a G2 d
Chemical Bonding
and Molecular Structure Methane 5 9 9 0 4 9 −0 5 0 7
Ethane 0 3 2 6 2 1 0 9 −0 2
Butane 0 7 −0 7 1 3 −0 8 −0 6
Pentane 0 7 −2 8 0 6 −0 5
Cyclopentane −11 9 −10 4 −5 6 4 0 −0 4
Cyclohexane −5 3 −9 0 −1 5 3 1 3 9
Cyclopropane −1 6
Cyclobutane −1 5
Bicyclo[1.1.0]butane −1 5
Bicyclo[2.2.1]heptane 2 1 −2 0 −1 3 8 8
Bicyclo[2.2.2]octane −2 2 −11 9 −3 7 10 7
Ethene 3 1 4 0 4 2 −2 4 0 3
Allene −1 6 0 6 1 5 −6 8 0 0 e
1,3-Butadiene 2 7 3 6 5 0 −2 9 0 5 f
Benzene 1 5 2 2 3 6 4 0 g
a. M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart, J. Am. Chem. Soc., 107, 3902 (1985).
b. M. J. S. Dewar and D. M. Storch, J. Am. Chem. Soc., 107, 3898 (1985).
c. J. J. P. Stewart, J. Comput. Chem., 10, 221 (1989).
d. J. A. Pople, M. Head-Gordon, D. J. Fox, K. Raghavachari, and L. A. Curtiss, J. Chem. Phys., 90, 5622 (1989);
L. A. Curtiss, K. Raghavachari, G. W. Trucks, and J. A. Pople, J. Chem. Phys., 94, 7221 (1991); L. A. Curtiss,
K. Raghavachari, P. C. Redfern, and J. Pople, J. Phys. Chem., 106, 1063 (1997).
e. D. W. Rogers and F. W. McLafferty, J. Phys. Chem., 99, 1375 (1993).
f. M. N. Glukhovtsev and S. Laiter, Theor. Chim. Acta, 92, 327 (1995).
g. A. Nicolaides and L. Radom, J. Phys. Chem., 98, 3092 (1994).
The results using HF/4-31G 63 and HF/6-31G ∗∗64 for some common substituents are
given below. They indicate that both electron-donating groups, such as amino and
methoxy, and electron-withdrawing groups, such as formyl and cyano, have a stabi-
lizing effect on double bonds. This is consistent with the implication of resonance that
there is a stabilizing interaction as a result of electron delocalization.
Stabilization (kcal/mol)
Substituent HF/4-31G HF/6-31G ∗∗
3 2 3 05
CH 3
11 2 7 20
NH 2
OH 6 6 6 43
6 1
OCH 3
F 0 99
Cl −0 54
CH=O 4 5
CN 2 4
−2 5
CF 3
The dipole moments of molecules depend on both the molecular dimensions and
the electron distribution. For example, Z-1,2-dichloroethene has a dipole moment of
1.90 D,whereas,owingtoitssymmetricalstructure,theE isomerhasnomoleculardipole.
63 A. Greenberg and T. A. Stevenson, J. Am. Chem. Soc., 107, 3488 (1985).
64
K. B. Wiberg and K. E. Laidig, J. Org. Chem., 57, 5092 (1992).