Page 50 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 50
Table 1.7. Energy Levels and Atomic Coefficients for HMOs of 1,3,5-Hexatriene 29
SECTION 1.2
orbital m j c 1 c 2 c 3 c 4 c 5 c 6
1 802 0 2319 0 4179 0 5211 0 5211 0 4179 0 2319 Molecular Orbital
1
Theory and Methods
1 247 0 4179 0 5211 0 2319 −0 2319 −0 5211 −0 4179
2
0 445 0 5211 0 2319 −0 4179 −0 4179 0 2319 0 5211
3
−0 445 0 5211 −0 2319 −0 4179 0 4179 0 2319 −0 5211
4
−1 247 0 4179 −0 5211 0 2319 0 2319 −0 5211 0 4179
5
−1 802 0 2319 −0 4179 0 5211 −0 5211 0 4179 −0 2319
6
in a circle with one point of the polygon at the bottom. The MO pattern corresponds
to each point of contact of the polygon and circle. If the circle is given a radius of 2 ,
the point of contact gives the coefficient of in the expression for the energy of the
MO. Compilations of HMO energy levels and atomic coefficients are available for a
number of conjugated systems. 42
What do we learn about molecules such as 1,3,5-hexatriene and benzene from
the HMO description of the orbitals?
1. The frontier MOs are identified and described. The frontier orbitals are the
highest occupied MO (HOMO) and the lowest unoccupied MO (LUMO). These orbitals
are intimately involved in chemical reactivity, because they are the most available
to electrophiles and nucleophiles, respectively. From the atomic coefficients, which
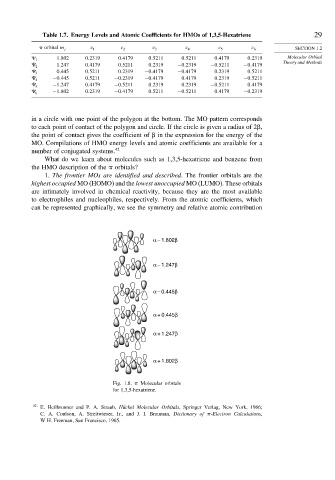
can be represented graphically, we see the symmetry and relative atomic contribution
α – 1.802β
α – 1.247β
α – 0.445β
α + 0.445β
α + 1.247β
α + 1.802β
Fig. 1.8. Molecular orbitals
for 1,3,5-hexatriene.
42
E. Heilbronner and P. A. Straub, Hückel Molecular Orbitals, Springer Verlag, New York, 1966;
C. A. Coulson, A. Streitwieser, Jr., and J. I. Brauman, Dictionary of -Electron Calculations,
W H. Freeman, San Francisco, 1965.