Page 43 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 43
22 E +
E
CHAPTER 1
O R O + R
Chemical Bonding
and Molecular Structure
-
O + R O R
O R
:
enhanced electron density at C(2)
E E E
+ +
OR O + R
stabilization of the cationic intermediate
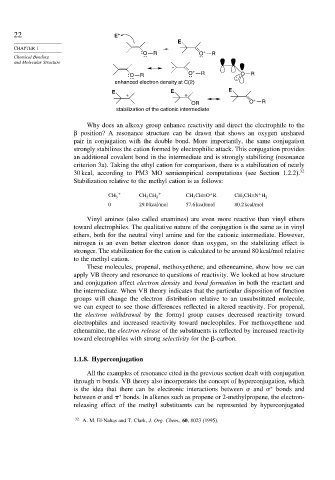
Why does an alkoxy group enhance reactivity and direct the electrophile to the
position? A resonance structure can be drawn that shows an oxygen unshared
pair in conjugation with the double bond. More importantly, the same conjugation
strongly stabilizes the cation formed by electrophilic attack. This conjugation provides
an additional covalent bond in the intermediate and is strongly stabilizing (resonance
criterion 3a). Taking the ethyl cation for comparison, there is a stabilization of nearly
30 kcal, according to PM3 MO semiempirical computations (see Section 1.2.2). 32
Stabilization relative to the methyl cation is as follows:
+ + CH 3 CH=O R +
+
CH 3 CH 3 CH 2 CH 3 CH=N H 2
0 29.0 kcal/mol 57.6 kcal/mol 80.2 kcal/mol
Vinyl amines (also called enamines) are even more reactive than vinyl ethers
toward electrophiles. The qualitative nature of the conjugation is the same as in vinyl
ethers, both for the neutral vinyl amine and for the cationic intermediate. However,
nitrogen is an even better electron donor than oxygen, so the stabilizing effect is
stronger. The stabilization for the cation is calculated to be around 80 kcal/mol relative
to the methyl cation.
These molecules, propenal, methoxyethene, and etheneamine, show how we can
apply VB theory and resonance to questions of reactivity. We looked at how structure
and conjugation affect electron density and bond formation in both the reactant and
the intermediate. When VB theory indicates that the particular disposition of function
groups will change the electron distribution relative to an unsubstituted molecule,
we can expect to see those differences reflected in altered reactivity. For propenal,
the electron withdrawal by the formyl group causes decreased reactivity toward
electrophiles and increased reactivity toward nucleophiles. For methoxyethene and
ethenamine, the electron release of the substituents is reflected by increased reactivity
toward electrophiles with strong selectivity for the -carbon.
1.1.8. Hyperconjugation
All the examples of resonance cited in the previous section dealt with conjugation
through bonds. VB theory also incorporates the concept of hyperconjugation, which
is the idea that there can be electronic interactions between and bonds and
∗
between and bonds. In alkenes such as propene or 2-methylpropene, the electron-
∗
releasing effect of the methyl substituents can be represented by hyperconjugated
32
A. M. El-Nahas and T. Clark, J. Org. Chem., 60, 8023 (1995).