Page 39 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 39
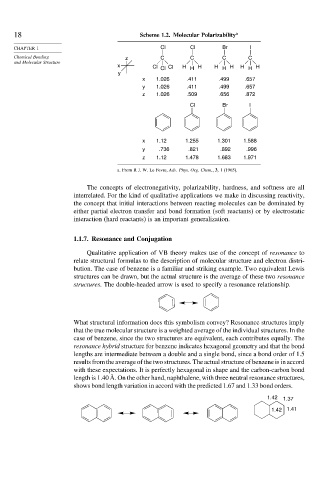
18 Scheme 1.2. Molecular Polarizability a
CHAPTER 1 Cl Cl Br I
Chemical Bonding z C C C C
and Molecular Structure
x Cl Cl Cl H H H H H H H H H
y
x 1.026 .411 .499 .657
y 1.026 .411 .499 .657
z 1.026 .509 .656 .872
Cl Br I
x 1.12 1.255 1.301 1.588
y .736 .821 .892 .996
z 1.12 1.478 1.683 1.971
a. From R J. W. Le Fevre, Adv. Phys. Org. Chem., 3, 1 (1965).
The concepts of electronegativity, polarizability, hardness, and softness are all
interrelated. For the kind of qualitative applications we make in discussing reactivity,
the concept that initial interactions between reacting molecules can be dominated by
either partial electron transfer and bond formation (soft reactants) or by electrostatic
interaction (hard reactants) is an important generalization.
1.1.7. Resonance and Conjugation
Qualitative application of VB theory makes use of the concept of resonance to
relate structural formulas to the description of molecular structure and electron distri-
bution. The case of benzene is a familiar and striking example. Two equivalent Lewis
structures can be drawn, but the actual structure is the average of these two resonance
structures. The double-headed arrow is used to specify a resonance relationship.
What structural information does this symbolism convey? Resonance structures imply
that the true molecular structure is a weighted average of the individual structures. In the
case of benzene, since the two structures are equivalent, each contributes equally. The
resonance hybrid structure for benzene indicates hexagonal geometry and that the bond
lengths are intermediate between a double and a single bond, since a bond order of 1.5
results from the average of the two structures. The actual structure of benzene is in accord
with these expectations. It is perfectly hexagonal in shape and the carbon-carbon bond
length is 1.40 Å. On the other hand, naphthalene, with three neutral resonance structures,
shows bond length variation in accord with the predicted 1.67 and 1.33 bond orders.
1.42 1.37
1.42 1.41