Page 40 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 40
Another important property of benzene is its thermodynamic stability, which is 19
greater than expected for either of the two resonance structures. It is much more
stable than noncyclic polyenes of similar structure, such as 1,3,5-hexatriene. What SECTION 1.1
is the origin of this additional stability, which is often called resonance stabilization Description of Molecular
Structure Using Valence
or resonance energy? The resonance structures imply that the electron density in Bond Concepts
benzene is equally distributed between the sets of adjacent carbon atoms. This is not
the case in acyclic polyenes. The electrons are evenly spread over the benzene ring, but
in the polyene they are more concentrated between alternating pairs of carbon atoms.
Average electron-electron repulsion is reduced in benzene. The difference in energy
is called the delocalization energy. The resonance structures for benzene describe
a particularly favorable bonding arrangement that leads to greater thermodynamic
stability. In keeping with our emphasis on structural theory as a means of describing
molecular properties, resonance describes, but does not cause, the extra stability.
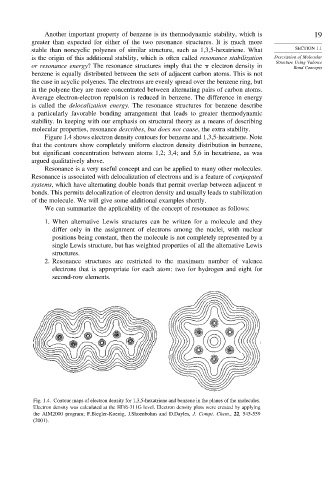
Figure 1.4 shows electron density contours for benzene and 1,3,5-hexatriene. Note
that the contours show completely uniform electron density distribution in benzene,
but significant concentration between atoms 1,2; 3,4; and 5,6 in hexatriene, as was
argued qualitatively above.
Resonance is a very useful concept and can be applied to many other molecules.
Resonance is associated with delocalization of electrons and is a feature of conjugated
systems, which have alternating double bonds that permit overlap between adjacent
bonds. This permits delocalization of electron density and usually leads to stabilization
of the molecule. We will give some additional examples shortly.
We can summarize the applicability of the concept of resonance as follows:
1. When alternative Lewis structures can be written for a molecule and they
differ only in the assignment of electrons among the nuclei, with nuclear
positions being constant, then the molecule is not completely represented by a
single Lewis structure, but has weighted properties of all the alternative Lewis
structures.
2. Resonance structures are restricted to the maximum number of valence
electrons that is appropriate for each atom: two for hydrogen and eight for
second-row elements.
Fig. 1.4. Contour maps of electron density for 1,3,5-hexatriene and benzene in the planes of the molecules.
Electron density was calculated at the HF/6-311G level. Electron density plots were created by applying
the AIM2000 program; F.Biegler-Koenig, J.Shoenbohm and D.Dayles, J. Compt. Chem., 22, 545-559
(2001).