Page 285 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 285
266 7221
CHAPTER 3 94,
Structural Effects on Chem.,
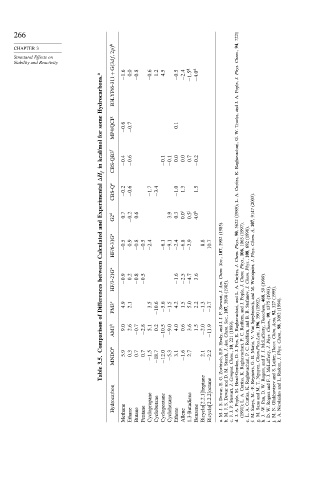
Stability and Reactivity B3LYP/6-311+G 3df 2p h −1 6 0.0 −0 8 −0 6 1.2 4.5 −0 5 −2 4 −1 5 d −4 0 d Phys.
Hydrocarbons. a J. Pople, A. J.
some MP4/QCI g −0 6 −0 7 0 1 and Trucks,
for W. G.
kcal/mol Raghavachari,
in CBS-QB3 f −0 4 −0 6 −0 1 −0 1 0 0 0 0 0 7 −0 2
H f K. Curtiss,
Experimental CBS-Q e −0 2 −0 6 −1 7 −3 4 −1 0 1 3 1 5 A. L. (1989); (2003). 9147
and G2 d 0.7 −0 2 0.6 3.9 0.3 0.0 i 0.5 j 4.0 k (1985). 5622 90, (1997). (1998). 107, A,
Calculated HF/6-31G a −0 5 0 9 −0 8 −0 5 −2 4 −6 1 −9 1 −2 4 −6 8 −2 9 8 8 10 7 3902 107, Phys., Chem. 1063 106, 692 108, Chem. Phys.
between HF/3-21G a −0 9 0 2 −0 8 −0 5 −1 6 −2 5 −4 7 3 6 Soc., Chem. Am. J. Curtiss, Phys., Chem. Phys., Chem. J. Waroquier, (1999).
Differences PM3 c 4 9 2 1 3 5 −10 6 −5 6 −1 5 4 2 1 5 5 0 2 2 −1 3 −3 7 J. Stewart, (1985). 3898 A. L. and J. Pople, J. J. Stefanov, M. and (1992). 59 468, (1993). 1375 (1995). 327 (1994).
of 9 0 2 6 5 1 0 2 4 0 0 6 3 6 1 5 P. J. J. 107, Soc., (1989). Raghavachari, and Redfern, B. B. and Speybroeck, 390 196, Theochem, 99, Chem., 92, Acta, 3092 98,
Comparison AM1 b −0 7 −2 8 −10 5 −9 0 −2 0 −11 9 and Healy, Chem. Am. 221 10, K. Fox, C. P. Redfern, C. Van V. Marin, Chem.Phys.Lett., McLafferty, Phys. J. Chim. Theor. Chem.,
3.5. MNDO a 5 9 0 3 0 7 0 7 −1 5 −18 7 −12 0 −5 3 3 1 −1 6 2 7 2 1 −2 2 E.F. Zoebisch, J. Storch, Chem., J. D. Raghavachari, P. B. G. J. F. and McLafferty, Laiter, Phys. J.
Table G. E. M. D. and Comput. J. Head-Gordon, K. Curtiss, Raghavachari, Reyniers, Nguyen, T. Rogers, W. J. F. S. and Radom, L. and
Hydrocarbon Bicyclo[2.2.1]heptane Bicyclo[2.2.2]octane Dewar, S. Dewar S. Stewart, P. M. Pople, A. L. K. Curtiss, -F. M. Saeys, M. and Sana D. Pan, and Rogers Glukhovtsev Nicolaides
Methane Ethane Butane Pentane Cyclopropane Cyclobutane Cyclopentane Cyclohexane Ethene Allene 1,3-Butadiene Benzene J. M. a. J. M. b. J. J. c. A. J. d. (1991); A. L. e. M. f. M. g. -W. J. h. W. D. i. N. M. j. A. k.