Page 287 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 287
o
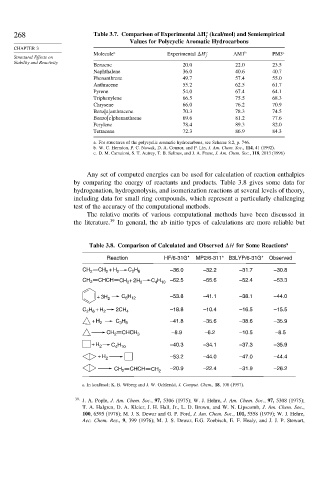
268 Table 3.7. Comparison of Experimental H (kcal/mol) and Semiempirical
f
Values for Polycyclic Aromatic Hydrocarbons
CHAPTER 3
Molecule a Experimental H o AM1 b PM3 c
Structural Effects on f
Stability and Reactivity
Benzene 20 0 22 0 23.5
Naphthalene 36 0 40 6 40.7
Phenanthrene 49 7 57 4 55.0
Anthracene 55 2 62 5 61.7
Pyrene 54 0 67 4 64.1
Triphenylene 66 5 75 5 68.3
Chrysene 66 0 76 2 70.9
Benz[a]anthracene 70 3 78 3 74.5
Benzo[c]phenanthrene 69 6 81 2 77.6
Perylene 78 4 89 3 82.0
Tetracene 72 3 86 9 84.3
a. For structures of the polycyclic aromatic hydrocarbons, see Scheme 8.2, p. 746.
b. W. C. Herndon, P. C. Nowak, D. A. Connor, and P. Lin, J. Am. Chem. Soc., 114, 41 (1992).
c. D. M. Camaioni, S. T. Autrey, T. B. Salinas, and J. A. Franz, J. Am. Chem. Soc., 118, 2013 (1996)
Any set of computed energies can be used for calculation of reaction enthalpies
by comparing the energy of reactants and products. Table 3.8 gives some data for
hydrogenation, hydrogenolysis, and isomerization reactions at several levels of theory,
including data for small ring compounds, which represent a particularly challenging
test of the accuracy of the computational methods.
The relative merits of various computational methods have been discussed in
the literature. 39 In general, the ab initio types of calculations are more reliable but
Table 3.8. Comparison of Calculated and Observed H for Some Reactions a
Reaction HF/6-31G* MP2/6-311* B3LYP/6-31G* Observed
CH 2 CH + H 2 C H –36.0 –32.2 –31.7 –30.8
2 6
2
CH 2 CHCH CH 2 + 2H 2 C 4 H 10 –62.5 –55.6 –52.4 –53.3
+ 3H 2 C H –53.8 –41.1 –38.1 –44.0
6 12
C H + H 2 2CH 4 –18.8 –10.4 –16.5 –15.5
2 6
+ H 2 C H –41.8 –35.6 –38.6 –35.9
3 8
–8.9 –6.2 –10.5 –8.5
CH 2 CHCH 3
+ H 2 C H –40.3 –34.1 –37.3 –35.9
4 10
+ H 2 –53.2 –44.0 –47.0 –44.4
CH 2 CHCH CH 2 –20.9 –22.4 –31.9 –26.2
a. In kcal/mol; K. B. Wiberg and J. W. Ochlerski, J. Comput. Chem., 18, 108 (1997).
39
J. A. Pople, J. Am. Chem. Soc., 97, 5306 (1975); W. J. Hehre, J. Am. Chem. Soc., 97, 5308 (1975);
T. A. Halgren, D. A. Kleier, J. H. Hall, Jr., L. D. Brown, and W. N. Lipscomb, J. Am. Chem. Soc.,
100, 6595 (1978); M. J. S. Dewar and G. P. Ford, J. Am. Chem. Soc., 101, 5558 (1979); W. J. Hehre,
Acc. Chem. Res., 9, 399 (1976); M. J. S. Dewar, E.G. Zoebisch, E. F. Healy, and J. J. P. Stewart,