Page 291 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 291
272 The temperature dependence of reactions is frequently expressed in terms of the
Arrhenius equation:
CHAPTER 3
Structural Effects on k = Ae −Ea/RT or ln k =−E /RT +ln A (3.24)
a
r
r
Stability and Reactivity
The E can be determined experimentally by measuring the reaction rate at several
a
temperatures over as wide a range as practical. A plot of ln k against 1/T then gives
r
a line of slope equal to –E /R. The value of E incorporates the H term and the
a
a
value of A reflects the entropy term. At constant pressure, E and H are related by
a
the expression
‡
H = E −RT (3.25)
a
‡
and S is related to A by
‡
S = R ln h/k T +ln A−1 (3.26)
B
‡
‡
The magnitude of H and S reflect transition state structure. Atomic positions
in the transition state do not correspond to their positions in the ground state. In
particular, the reacting bonds are partially formed or partially broken. The energy
required for bond reorganization is reflected in the higher energy content of the
‡
activated complex and corresponds to the enthalpy of activation H . The entropy of
‡
activation, S , is a measure of the degree of organization resulting from the formation
of the activated complex. If translational, vibrational, or rotational degrees of freedom
are lost in going to the transition state, there is a decrease in the total entropy of
the system. Conversely, an increase of translational, vibrational, or rotational degrees
of freedom results in a positive entropy of activation. For reactions in solution, the
organization of solvent contributes to the entropy of activation.
Wide variation in enthalpy and entropy of activation for different reaction systems
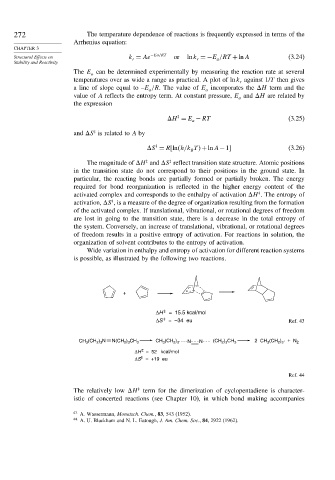
is possible, as illustrated by the following two reactions.
+
‡
ΔH = 15.5 kcal/mol
‡
ΔS = –34 eu Ref. 43
CH 3 (CH 2 ) 3 N N(CH 2 ) 3 CH 3 CH 3 (CH 2 ) 3 N N (CH 2 ) 3 CH 3 2 CH 3 (CH 2 ) 3 · + N 2
‡
ΔH = 52 kcal/mol
‡
ΔS = +19 eu
Ref. 44
‡
The relatively low H term for the dimerization of cyclopentadiene is character-
istic of concerted reactions (see Chapter 10), in which bond making accompanies
43 A. Wassermann, Monatsch. Chem., 83, 543 (1952).
44
A. U. Blackham and N. L. Eatough, J. Am. Chem. Soc., 84, 2922 (1962).