Page 292 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 292
‡
bond breaking. It differs markedly from H for the thermal decomposition of 273
1 1 -azobutane, in which the rate-determining step is a homolytic cleavage of a C−N
bond, with little new bond making to compensate for the energy cost of the bond SECTION 3.2
breaking. The entropy of activation, on the other hand, is more favorable in the 1 1 - Chemical Kinetics
azobutane decomposition, since a translational degree of freedom is being gained in
the TS as the molecular fragments separate. The dimerization of cyclopentadiene is
accompanied by a very negative entropy of activation because of the loss of transla-
tional and rotational degrees of freedom in formation of the highly ordered cyclic TS.
The two reacting molecules must a attain a specific orientation to permit the bonding
interactions that occur as the TS is approached. Unimolecular reactions that take place
by way of cyclic transition states also typically have negative entropies of activation
because of the loss of rotational degrees of freedom associated with the highly ordered
‡
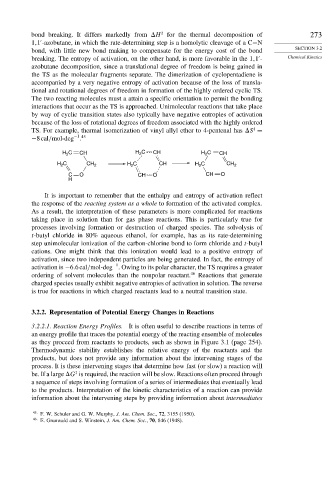
TS. For example, thermal isomerization of vinyl allyl ether to 4-pentenal has S =
−1 45
−8cal/mol-deg .
C H C CH H C
H 2 CH 2 2 CH
H C CH 2 H C CH H C CH 2
2
2
2
C O CH O CH O
H
It is important to remember that the enthalpy and entropy of activation reflect
the response of the reacting system as a whole to formation of the activated complex.
As a result, the interpretation of these parameters is more complicated for reactions
taking place in solution than for gas phase reactions. This is particularly true for
processes involving formation or destruction of charged species. The solvolysis of
t-butyl chloride in 80% aqueous ethanol, for example, has as its rate-determining
step unimolecular ionization of the carbon-chlorine bond to form chloride and t-butyl
cations. One might think that this ionization would lead to a positive entropy of
activation, since two independent particles are being generated. In fact, the entropy of
−1
activation is −6 6cal/mol-deg . Owing to its polar character, the TS requires a greater
ordering of solvent molecules than the nonpolar reactant. 46 Reactions that generate
charged species usually exhibit negative entropies of activation in solution. The reverse
is true for reactions in which charged reactants lead to a neutral transition state.
3.2.2. Representation of Potential Energy Changes in Reactions
3.2.2.1. Reaction Energy Profiles. It is often useful to describe reactions in terms of
an energy profile that traces the potential energy of the reacting ensemble of molecules
as they proceed from reactants to products, such as shown in Figure 3.1 (page 254).
Thermodynamic stability establishes the relative energy of the reactants and the
products, but does not provide any information about the intervening stages of the
process. It is these intervening stages that determine how fast (or slow) a reaction will
‡
be. If a large G is required, the reaction will be slow. Reactions often proceed through
a sequence of steps involving formation of a series of intermediates that eventually lead
to the products. Interpretation of the kinetic characteristics of a reaction can provide
information about the intervening steps by providing information about intermediates
45 F. W. Schuler and G. W. Murphy, J. Am. Chem. Soc., 72, 3155 (1950).
46
E. Grunwald and S. Winstein, J. Am. Chem. Soc., 70, 846 (1948).