Page 286 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 286
2
3
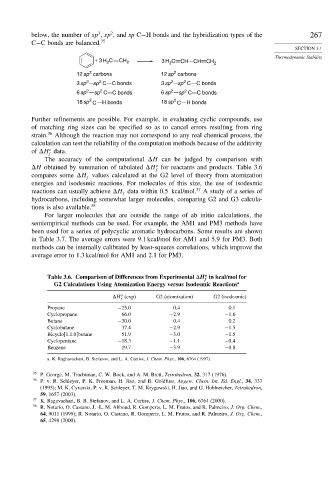
below, the number of sp , sp , and sp C−H bonds and the hybridization types of the 267
C−C bonds are balanced. 35
SECTION 3.1
Thermodynamic Stability
+ 3 H C CH 2 3 H 2 C CH CH CH 2
2
2
2
12 sp carbons 12 sp carbons
2 2 2 2
3 sp sp C C bonds 3 sp sp C C bonds
2 2 2 2
6 sp sp C C bonds 6 sp sp C C bonds
2 2
18 sp C H bonds 18 sp C H bonds
Further refinements are possible. For example, in evaluating cyclic compounds, use
of matching ring sizes can be specified so as to cancel errors resulting from ring
strain. 36 Although the reaction may not correspond to any real chemical process, the
calculation can test the reliability of the computation methods because of the additivity
o
of H data.
f
The accuracy of the computational H can be judged by comparison with
o
H obtained by summation of tabulated H for reactants and products. Table 3.6
f
compares some H values calculated at the G2 level of theory from atomization
f
energies and isodesmic reactions. For molecules of this size, the use of isodesmic
reactions can usually achieve H data within 0.5 kcal/mol. 37 A study of a series of
f
hydrocarbons, including somewhat larger molecules, comparing G2 and G3 calcula-
tions is also available. 38
For larger molecules that are outside the range of ab initio calculations, the
semiempirical methods can be used. For example, the AM1 and PM3 methods have
been used for a series of polycyclic aromatic hydrocarbons. Some results are shown
in Table 3.7. The average errors were 9.1 kcal/mol for AM1 and 5.9 for PM3. Both
methods can be internally calibrated by least-squares correlations, which improve the
average error to 1.3 kcal/mol for AM1 and 2.1 for PM3.
o
Table 3.6. Comparison of Differences from Experimental H in kcal/mol for
f
G2 Calculations Using Atomization Energy versus Isodesmic Reactions a
o
H (exp) G2 (atomization) G2 (isodesmic)
f
Propane −25 0 0.4 0.1
Cyclopropane 66.0 −2 9 −1 6
Butane −30 0 0.4 0.2
Cyclobutane 37.4 −2 9 −1 5
Bicyclo[1.1.0]butane 51.9 −3 0 −1 5
Cyclopentane −18 3 −1 1 −0 4
Benzene 19.7 −3 9 −0 8
a. K. Raghavachari, B. Stefanov, and L. A. Curtiss, J. Chem. Phys., 106, 6764 (1997).
35 P. George, M. Trachtman, C. W. Bock, and A. M. Brett, Tetrahedron, 32, 317 (1976).
36
P. v. R. Schleyer, P. K. Freeman, H. Jiao, and B. Goldfuss, Angew. Chem. Int. Ed. Engl., 34, 337
(1995); M. K. Cyranski, P. v. R. Schleyer, T. M. Krygowski, H. Jiao, and G. Hohlneicher, Tetrahedron,
59, 1657 (2003).
37 K. Ragavachari, B. B. Stefanov, and L. A. Curtiss, J. Chem. Phys., 106, 6764 (2000).
38
R. Notario, O. Castano, J. -L. M. Abboud, R. Gomperts, L. M. Frutos, and R. Palmeiro, J. Org. Chem.,
64, 9011 (1999); R. Notario, O. Castano, R. Gomperts, L. M. Frutos, and R. Palmeiro, J. Org. Chem.,
65, 4298 (2000).