Page 539 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 539
520 directing, whereas ethyl groups are anti directing. These results were attributed to
differential polarization of the faces of the bond (see Section 2.4). Similar, but much
CHAPTER 5 weaker, directive effects are seen for epoxidation and hydroboration.
Polar Addition
and Elimination anti H C syn
2
Reactions X syn anti
CO CH >95 >5
2 3
CH OCH 40 60
X 2 3
X CH CH 3 17 83
2
A broad mechanistic interpretation that can encompass most of these results
suggests that the Hg 2+ ion is rather loosely bound prior to the rate-determining nucle-
ophilic addition. The bonding is weaker at the carbon best able to accommodate positive
charge, resulting in Markovnikov regioselectivity. If there is little steric hindrance,
anti addition of a nucleophile is preferred, but the syn mechanism is available. The
syn mechanism, at least as modeled in the gas phase, seems to involve migration of a
ligand from Hg 2+ to carbon. It is the nucleophilic capture that determines the regio-
chemistry and stereochemistry of the product, since the mercuration step is reversible.
It should also be noted that oxymercuration has a different electronic pattern from
most of the other electrophilic additions in that the Hg-substituted carbon becomes
negatively charged as bonding occurs (see Figure 5.5). This is also true in hydrobo-
ration, although less so, whereas halogenation, sulfenylation, and selenylation result
in placing a more electronegative substituent on both carbons of the double bond.
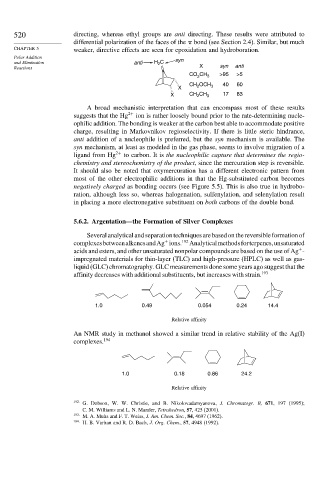
5.6.2. Argentation—the Formation of Silver Complexes
Severalanalyticalandseparationtechniquesarebasedonthereversibleformationof
+
complexesbetweenalkenesandAg ions. 192 Analyticalmethodsforterpenes,unsaturated
+
acids and esters, and other unsaturated nonpolar compounds are based on the use of Ag -
impregnated materials for thin-layer (TLC) and high-pressure (HPLC) as well as gas-
liquid (GLC) chromatography. GLC measurements done some years ago suggest that the
affinity decreases with additional substituents, but increases with strain. 193
1.0 0.49 0.054 0.24 14.4
Relative affinity
An NMR study in methanol showed a similar trend in relative stability of the Ag(I)
complexes. 194
1.0 0.18 0.86 24.2
Relative affinity
192
G. Dobson, W. W. Christie, and B. Nikolovadamyanova, J. Chromatogr. B, 671, 197 (1995);
C. M. Williams and L. N. Mander, Tetrahedron, 57, 425 (2001).
193 M. A. Muhs and F. T. Weiss, J. Am. Chem. Soc., 84, 4697 (1962).
194 H. B. Varhan and R. D. Bach, J. Org. Chem., 57, 4948 (1992).