Page 537 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 537
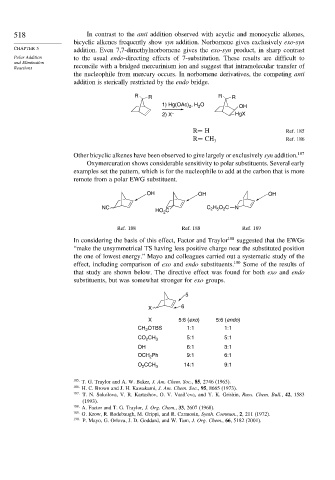
518 In contrast to the anti addition observed with acyclic and monocyclic alkenes,
bicyclic alkenes frequently show syn addition. Norbornene gives exclusively exo-syn
CHAPTER 5 addition. Even 7,7-dimethylnorbornene gives the exo-syn product, in sharp contrast
Polar Addition to the usual endo-directing effects of 7-substitution. These results are difficult to
and Elimination
Reactions reconcile with a bridged mercurinium ion and suggest that intramolecular transfer of
the nucleophile from mercury occurs. In norbornene derivatives, the competing anti
addition is sterically restricted by the endo bridge.
R R R R
1) Hg(OAc) , H O OH
2
2
2) X – HgX
R= H Ref. 185
R= CH 3 Ref. 186
Other bicyclic alkenes have been observed to give largely or exclusively syn addition. 187
Oxymercuration shows considerable sensitivity to polar substituents. Several early
examples set the pattern, which is for the nucleophile to add at the carbon that is more
remote from a polar EWG substituent.
OH OH OH
NC C H O C N
2 5
2
HO C
2
Ref. 188 Ref. 188 Ref. 189
In considering the basis of this effect, Factor and Traylor 188 suggested that the EWGs
“make the unsymmetrical TS having less positive charge near the substituted position
the one of lowest energy.” Mayo and colleagues carried out a systematic study of the
effect, including comparison of exo and endo substituents. 190 Some of the results of
that study are shown below. The directive effect was found for both exo and endo
substituents, but was somewhat stronger for exo groups.
5
X 6
X 5:6 (exo) 5:6 (endo)
CH OTBS 1:1 1:1
2
CO CH 3 5:1 5:1
2
OH 6:1 3:1
OCH Ph 9:1 6:1
2
O 2 CCH 3 14:1 9:1
185
T. G. Traylor and A. W. Baker, J. Am. Chem. Soc., 85, 2746 (1963).
186 H. C. Brown and J. H. Kawakami, J. Am. Chem. Soc., 95, 8665 (1973).
187 T. N. Sokolova, V. R. Kartashov, O. V. Vasil’eva, and Y. K. Grishin, Russ. Chem. Bull., 42, 1583
(1993).
188
A. Factor and T. G. Traylor, J. Org. Chem., 33, 2607 (1968).
189 G. Krow, R. Rodebaugh, M. Grippi, and R. Carmosin, Synth. Commun., 2, 211 (1972).
190
P. Mayo, G. Orlova, J. D. Goddard, and W. Tam, J. Org. Chem., 66, 5182 (2001).