Page 532 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 532
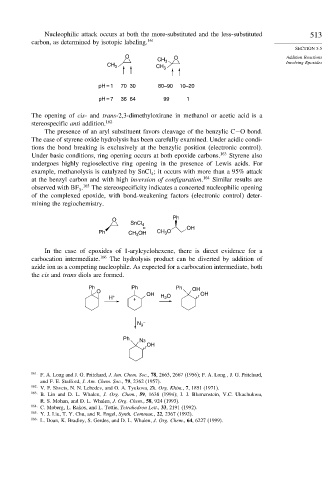
Nucleophilic attack occurs at both the more-substituted and the less-substituted 513
carbon, as determined by isotopic labeling. 161
SECTION 5.5
O O Addition Reactions
CH 3
CH 3 CH 3 Involving Epoxides
pH = 1 70 30 80–90 10–20
pH = 7 36 64 99 1
The opening of cis- and trans-2,3-dimethyloxirane in methanol or acetic acid is a
stereospecific anti addition. 162
The presence of an aryl substituent favors cleavage of the benzylic C−O bond.
The case of styrene oxide hydrolysis has been carefully examined. Under acidic condi-
tions the bond breaking is exclusively at the benzylic position (electronic control).
Under basic conditions, ring opening occurs at both epoxide carbons. 163 Styrene also
undergoes highly regioselective ring opening in the presence of Lewis acids. For
example, methanolysis is catalyzed by SnCl ; it occurs with more than a 95% attack
4
at the benzyl carbon and with high inversion of configuration. 164 Similar results are
observed with BF . 165 The stereospecificity indicates a concerted nucleophilic opening
3
of the complexed epoxide, with bond-weakening factors (electronic control) deter-
mining the regiochemistry.
O Ph
SnCl 4
OH
Ph CH OH CH 3 O
3
In the case of epoxides of 1-arylcyclohexene, there is direct evidence for a
carbocation intermediate. 166 The hydrolysis product can be diverted by addition of
azide ion as a competing nucleophile. As expected for a carbocation intermediate, both
the cis and trans diols are formed.
Ph Ph Ph OH
O OH OH
H + + H O
2
N 3 –
Ph N3
OH
161 F. A. Long and J. G. Pritchard, J. Am. Chem. Soc., 78, 2663, 2667 (1956); F. A. Long , J. G. Pritchard,
and F. E. Stafford, J. Am. Chem. Soc., 79, 2362 (1957).
162
V. F. Shvets, N. N. Lebedev, and O. A. Tyukova, Zh. Org. Khim., 7, 1851 (1971).
163
B. Lin and D. L. Whalen, J. Org. Chem., 59, 1638 (1994); J. J. Blumenstein, V.C. Ukachukwu,
R. S. Mohan, and D. L. Whalen, J. Org. Chem., 58, 924 (1993).
164 C. Moberg, L. Rakos, and L. Tottie, Tetrahedron Lett., 33, 2191 (1992).
165 Y. J. Liu, T. Y. Chu, and R. Engel, Synth. Commun., 22, 2367 (1992).
166
L. Doan, K. Bradley, S. Gerdes, and D. L. Whalen, J. Org. Chem., 64, 6227 (1999).