Page 533 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 533
514 Similarly, comparison of the epoxide of 1,2-dihydronaphthalene and its 6-methoxy
analog provided interesting contrasts. The product was partitioned into the compo-
CHAPTER 5 nents formed by the acid-catalyzed and uncatalyzed mechanisms. 167 The unsubstituted
Polar Addition compound gives the trans diol exclusively, indicating participation of the nucle-
and Elimination
Reactions ophile in the ring opening. The 6-methoxy derivative gives a substantial amount of
a rearrangement product and the diol is a mixture of the cis and trans stereoisomers.
These differences indicate that the more stabilized carbocation has a significant
lifetime.
OH
O O
OH
+
X X X
X cis trans
H
+
H – catalyzed 6 94 0
uncatalyzed 0 100 0
O
CH 3
+
H – catalyzed 81 19 <1
uncatalyzed 17 7 76
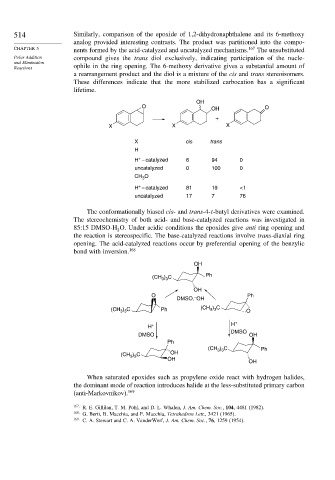
The conformationally biased cis- and trans-4-t-butyl derivatives were examined.
The stereochemistry of both acid- and base-catalyzed reactions was investigated in
85:15 DMSO-H O. Under acidic conditions the epoxides give anti ring opening and
2
the reaction is stereospecific. The base-catalyzed reactions involve trans-diaxial ring
opening. The acid-catalyzed reactions occur by preferential opening of the benzylic
bond with inversion. 168
OH
(CH ) C Ph
3 3
OH
O – Ph
DMSO, OH
) C
3 3
(CH 3 3 Ph (CH ) C O
H + H +
DMSO
DMSO OH
Ph
(CH ) C Ph
OH 3 3
(CH ) C
3 3
OH
OH
When saturated epoxides such as propylene oxide react with hydrogen halides,
the dominant mode of reaction introduces halide at the less-substituted primary carbon
(anti-Markovnikov). 169
167 R. E. Gillilan, T. M. Pohl, and D. L. Whalen, J. Am. Chem. Soc., 104, 4481 (1982).
168 G. Berti, B. Macchia, and F. Macchia, Tetrahedron Lett., 3421 (1965).
169
C. A. Stewart and C. A. VanderWerf, J. Am. Chem. Soc., 76, 1259 (1954).