Page 528 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 528
509
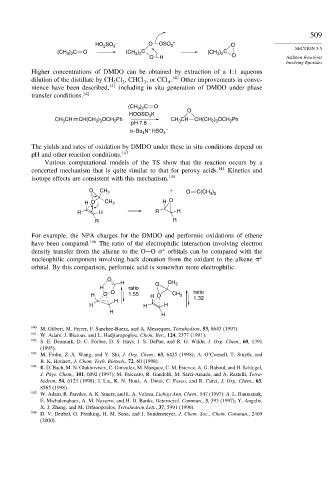
HO SO 3 – O OSO 3 – O
2
(CH ) C O (CH ) C (CH ) C SECTION 5.5
3 2
3 2
3 2
O H O Addition Reactions
Involving Epoxides
Higher concentrations of DMDO can be obtained by extraction of a 1:1 aqueous
dilution of the distillate by CH Cl , CHCl , or CCl . 140 Other improvements in conve-
4
3
2
2
nience have been described, 141 including in situ generation of DMDO under phase
transfer conditions. 142
(CH ) C O
3 2
O
HOOSO 3 K
CH CH CH(CH ) OCH Ph pH 7.8 CH 3 CH CH(CH ) OCH Ph
2
2 3
3
2 3
2
+
–
n –Bu N HSO 4
4
The yields and rates of oxidation by DMDO under these in situ conditions depend on
pH and other reaction conditions. 143
Various computational models of the TS show that the reaction occurs by a
concerted mechanism that is quite similar to that for peroxy acids. 144 Kinetics and
isotope effects are consistent with this mechanism. 145
O CH 3 + O C(CH )
3 2
H O CH 3 H O
R H R H
R R
For example, the NPA charges for the DMDO and performic oxidations of ethene
have been compared. 146 The ratio of the electrophilic interaction involving electron
density transfer from the alkene to the O−O
orbitals can be compared with the
∗
nucleophilic component involving back donation from the oxidant to the alkene ∗
orbital. By this comparison, performic acid is somewhat more electrophilic.
O
H O CH 3
H ratio
H O O 1.55 H O CH 3 ratio
H 1.32
H H H
H
H
140 M. Gilbert, M. Ferrer, F. Sanchez-Baeza, and A. Messequer, Tetrahedron, 53, 8643 (1997).
141
W. Adam, J. Bialoas, and L. Hadjiaropoglou, Chem. Ber., 124, 2377 (1991).
142 S. E. Denmark, D. C. Forbes, D. S. Hays, J. S. DePue, and R. G. Wilde, J. Org. Chem., 60, 1391
(1995).
143 M. Frohn, Z.-X. Wang, and Y. Shi, J. Org. Chem., 63, 6425 (1998); A. O’Connell, T. Smyth, and
B. K. Hodnett, J. Chem. Tech. Biotech., 72, 60 (1998).
144
R. D. Bach, M. N. Glukhovtsev, C. Gonzalez, M. Marquez, C. M. Estevez, A. G. Baboul, and H. Schlegel,
J. Phys. Chem., 101, 6092 (1997); M. Freccero, R. Gandolfi, M. Sarzi-Amade, and A. Rastelli, Tetra-
hedron, 54, 6123 (1998); J. Liu, K. N. Houk, A. Dinoi, C. Fusco, and R. Curci, J. Org. Chem., 63,
8565 (1998).
145 W. Adam, R. Paredes, A. K. Smerz, and L. A. Veloza, Liebigs Ann. Chem., 547 (1997); A. L. Baumstark,
E. Michalenabaez, A. M. Navarro, and H. D. Banks, Heterocycl. Commun., 3, 393 (1997); Y. Angelis,
X. J. Zhang, and M. Orfanopoulos, Tetrahedron Lett., 37, 5991 (1996).
146
D. V. Deubel, G. Frenking, H. M. Senn, and J. Sundermeyer, J. Chem. Soc., Chem. Commun., 2469
(2000).