Page 524 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 524
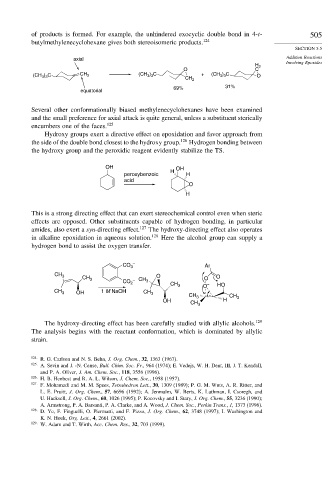
of products is formed. For example, the unhindered exocyclic double bond in 4-t- 505
butylmethylenecyclohexane gives both stereoisomeric products. 124
SECTION 5.5
Addition Reactions
axial
Involving Epoxides
H 2
O C
(CH 3 ) 3 C CH 2 (CH 3 ) 3 C + (CH 3 ) 3 C O
CH 2
69% 31%
equatorial
Several other conformationally biased methylenecyclohexanes have been examined
and the small preference for axial attack is quite general, unless a substituent sterically
encumbers one of the faces. 125
Hydroxy groups exert a directive effect on epoxidation and favor approach from
the side of the double bond closest to the hydroxy group. 126 Hydrogen bonding between
the hydroxy group and the peroxidic reagent evidently stabilize the TS.
OH OH
H
peroxybenzoic H
acid
O
H
This is a strong directing effect that can exert stereochemical control even when steric
effects are opposed. Other substituents capable of hydrogen bonding, in particular
amides, also exert a syn-directing effect. 127 The hydroxy-directing effect also operates
in alkaline epoxidation in aqueous solution. 128 Here the alcohol group can supply a
hydrogen bond to assist the oxygen transfer.
–
CO 3 Ar
CH 3 CH O O
3
CO 2 – CH 3 CH 3 O – HO
CH 3 OH 1 M NaOH CH O
3
CH 3 CH 3
OH CH 3 H
The hydroxy-directing effect has been carefully studied with allylic alcohols. 129
The analysis begins with the reactant conformation, which is dominated by allylic
strain.
124
R. G. Carlson and N. S. Behn, J. Org. Chem., 32, 1363 (1967).
125 A. Sevin and J. -N. Cense, Bull. Chim. Soc. Fr., 964 (1974); E. Vedejs, W. H. Dent, III, J. T. Kendall,
and P. A. Oliver, J. Am. Chem. Soc., 118, 3556 (1996).
126 H. B. Henbest and R. A. L. Wilson, J. Chem. Soc., 1958 (1957).
127
F. Mohamadi and M. M. Spees, Tetrahedron Lett., 30, 1309 (1989); P. G. M. Wuts, A. R. Ritter, and
L. E. Pruitt, J. Org. Chem., 57, 6696 (1992); A. Jenmalm, W. Berts, K. Luthman, I. Csoregh, and
U. Hacksell, J. Org. Chem., 60, 1026 (1995); P. Kocovsky and I. Stary, J. Org. Chem., 55, 3236 (1990);
A. Armstrong, P. A. Barsanti, P. A. Clarke, and A. Wood, J. Chem. Soc., Perkin Trans., 1, 1373 (1996).
128 D. Ye, F. Finguelli, O. Piermatti, and F. Pizzo, J. Org. Chem., 62, 3748 (1997); I. Washington and
K. N. Houk, Org. Lett., 4, 2661 (2002).
129
W. Adam and T. Wirth, Acc. Chem. Res., 32, 703 (1999).