Page 520 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 520
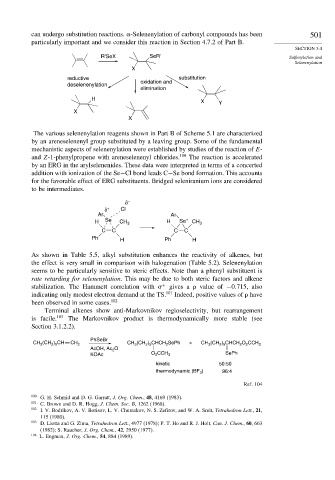
can undergo substitution reactions. -Selenenylation of carbonyl compounds has been 501
particularly important and we consider this reaction in Section 4.7.2 of Part B.
SECTION 5.4
R'SeX SeR' Sulfenylation and
Selenenylation
X
reductive substitution
oxidation and
deselenenylation
elimination
H
X Y
X
X
The various selenenylation reagents shown in Part B of Scheme 5.1 are characterized
by an areneselenenyl group substituted by a leaving group. Some of the fundamental
mechanistic aspects of selenenylation were established by studies of the reaction of E-
and Z-1-phenylpropene with areneselenenyl chlorides. 100 The reaction is accelerated
by an ERG in the arylselenenides. These data were interpreted in terms of a concerted
addition with ionization of the Se−Cl bond leads C−Se bond formation. This accounts
for the favorable effect of ERG substituents. Bridged seleniranium ions are considered
to be intermediates.
δ –
δ + Cl
Ar Ar
H Se CH 3 H Se + CH 3
C C C C
Ph H Ph H
As shown in Table 5.5, alkyl substitution enhances the reactivity of alkenes, but
the effect is very small in comparison with halogenation (Table 5.2). Selenenylation
seems to be particularly sensitive to steric effects. Note than a phenyl substituent is
rate retarding for selenenylation. This may be due to both steric factors and alkene
stabilization. The Hammett correlation with
+ gives a
value of −0 715, also
indicating only modest electron demand at the TS. 101 Indeed, positive values of
have
been observed in some cases. 102
Terminal alkenes show anti-Markovnikov regioselectivity, but rearrangement
is facile. 103 The Markovnikov product is thermodynamically more stable (see
Section 3.1.2.2).
PhSeBr
CH 3 (CH 2 ) 5 CH CH 2 CH 3 (CH 2 ) 5 CHCH 2 SePh + CH 3 (CH 2 ) 5 CHCH 2 O 2 CCH 3
AcOH, Ac 2 O
KOAc O 2 CCH 3 SePh
kinetic 50:50
thermodynamic (BF 3 ) 96:4
Ref. 104
100 G. H. Schmid and D. G. Garratt, J. Org. Chem., 48, 4169 (1983).
101
C. Brown and D. R. Hogg, J. Chem. Soc. B, 1262 (1968).
102
I. V. Bodrikov, A. V. Borisov, L. V. Chumakov, N. S. Zefirov, and W. A. Smit, Tetrahedron Lett., 21,
115 (1980).
103 D. Liotta and G. Zima, Tetrahedron Lett., 4977 (1978); P. T. Ho and R. J. Holt, Can. J. Chem., 60, 663
(1982); S. Raucher, J. Org. Chem., 42, 2950 (1977).
104
L. Engman, J. Org. Chem., 54, 884 (1989).