Page 523 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 523
504 The magnesium salt of monoperoxyphthalic acid is an alternative. 112 Peroxyacetic
acid, peroxybenzoic acid, and peroxytrifluoroacetic acid also are used frequently for
CHAPTER 5
epoxidation. All of the peroxycarboxylic acids are potentially explosive materials and
Polar Addition require careful handling. Potassium hydrogen peroxysulfate, which is sold commer-
and Elimination
113
Reactions cially as Oxone , is a convenient reagent for epoxidations that can be done in
aqueous solution. 114
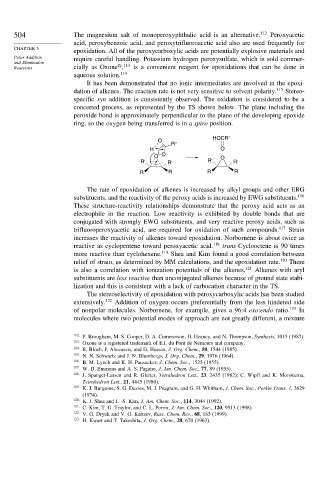
It has been demonstrated that no ionic intermediates are involved in the epoxi-
dation of alkenes. The reaction rate is not very sensitive to solvent polarity. 115 Stereo-
specific syn addition is consistently observed. The oxidation is considered to be a
concerted process, as represented by the TS shown below. The plane including the
peroxide bond is approximately perpendicular to the plane of the developing epoxide
ring, so the oxygen being transferred is in a spiro position.
HOCR"
O
R"
H O
O O O
R' R' R' R'
R R R R
The rate of epoxidation of alkenes is increased by alkyl groups and other ERG
substituents, and the reactivity of the peroxy acids is increased by EWG substituents. 116
These structure-reactivity relationships demonstrate that the peroxy acid acts as an
electrophile in the reaction. Low reactivity is exhibited by double bonds that are
conjugated with strongly EWG substituents, and very reactive peroxy acids, such as
trifluoroperoxyacetic acid, are required for oxidation of such compounds. 117 Strain
increases the reactivity of alkenes toward epoxidation. Norbornene is about twice as
reactive as cyclopentene toward peroxyacetic acid. 118 trans-Cyclooctene is 90 times
more reactive than cyclohexene. 119 Shea and Kim found a good correlation between
relief of strain, as determined by MM calculations, and the epoxidation rate. 120 There
is also a correlation with ionization potentials of the alkenes. 121 Alkenes with aryl
substituents are less reactive than unconjugated alkenes because of ground state stabi-
lization and this is consistent with a lack of carbocation character in the TS.
The stereoselectivity of epoxidation with peroxycarboxylic acids has been studied
extensively. 122 Addition of oxygen occurs preferentially from the less hindered side
of nonpolar molecules. Norbornene, for example, gives a 96:4 exo:endo ratio. 123 In
molecules where two potential modes of approach are not greatly different, a mixture
112 P. Brougham, M. S. Cooper, D. A. Cummerson, H. Heaney, and N. Thompson, Synthesis, 1015 (1987).
113
Oxone is a registered trademark of E.I. du Pont de Nemours and company.
114 R. Bloch, J. Abecassis, and D. Hassan, J. Org. Chem., 50, 1544 (1985).
115
N. N. Schwartz and J. N. Blumbergs, J. Org. Chem., 29, 1976 (1964).
116
B. M. Lynch and K. H. Pausacker, J. Chem. Soc., 1525 (1955).
117 W. D. Emmons and A. S. Pagano, J. Am. Chem. Soc., 77, 89 (1955).
118
J. Spanget-Larsen and R. Gleiter, Tetrahedron Lett., 23, 2435 (1982); C. Wipff and K. Morokuma,
Tetrahedron Lett., 21, 4445 (1980).
119 K. J. Burgoine, S. G. Davies, M. J. Peagram, and G. H. Whitham, J. Chem. Soc., Perkin Trans. 1, 2629
(1974).
120
K. J. Shea and J. -S. Kim, J. Am. Chem. Soc., 114, 3044 (1992).
121
C. Kim, T. G. Traylor, and C. L. Perrin, J. Am. Chem. Soc., 120, 9513 (1998).
122 V. G. Dryuk and V. G. Kartsev, Russ. Chem. Rev., 68, 183 (1999).
123
H. Kwart and T. Takeshita, J. Org. Chem., 28, 670 (1963).