Page 519 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 519
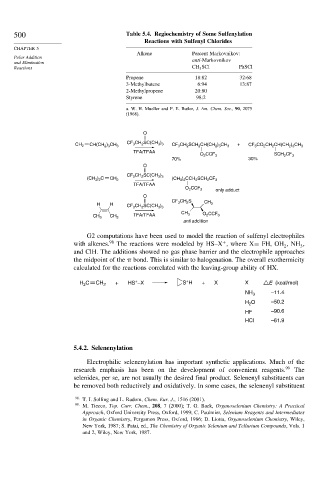
500 Table 5.4. Regiochemistry of Some Sulfenylation
Reactions with Sulfenyl Chlorides
CHAPTER 5
Alkene Percent Markovnikov:
Polar Addition
and Elimination anti-Markovnikov
Reactions CH 3 SCl PhSCl
Propene 18:82 32:68
3-Methylbutene 6:94 13:87
2-Methylpropene 20:80
Styrene 98:2
a. W. H. Mueller and P. E. Butler, J. Am. Chem. Soc., 90, 2075
(1968).
O
CF 3 CH 2 SC(CH 3 ) 3 +
CH 2 CH(CH 2 ) 3 CH 3 CF 3 CH 2 SCH 2 CH(CH 2 ) 3 CH 3 CF 3 CO 2 CH 2 CH(CH 2 ) 3 CH 3
TFA/TFAA
O 2 CCF 3 SCH 2 CF 3
70% 30%
O
CF 3 CH 2 SC(CH 3 ) 3
(CH 3 ) 2 C CH 2 (CH 3 ) 2 CCH 2 SCH 2 CF 3
TFA/TFAA
only adduct
O 2 CCF 3
O
CF 3 CH 2 S
H H CH 3
CF 3 CH 2 SC(CH 3 ) 3
TFA/TFAA CH 3 O 2 CCF 3
CH 3 CH 3
anti addition
G2 computations have been used to model the reaction of sulfenyl electrophiles
+
with alkenes. 98 The reactions were modeled by HS–X , where X= FH, OH NH ,
3
2
and ClH. The additions showed no gas phase barrier and the electrophile approaches
the midpoint of the bond. This is similar to halogenation. The overall exothermicity
calculated for the reactions correlated with the leaving-group ability of HX.
+
+
C + HS –X S H + X X E (kcal/mol)
H 2 CH 2
–11.4
NH 3
H O –50.2
2
HF –90.6
HCl –61.9
5.4.2. Selenenylation
Electrophilic selenenylation has important synthetic applications. Much of the
research emphasis has been on the development of convenient reagents. 99 The
selenides, per se, are not usually the desired final product. Selenenyl substituents can
be removed both reductively and oxidatively. In some cases, the selenenyl substituent
98 T. I. Solling and L. Radom, Chem. Eur. J., 1516 (2001).
99
M. Tiecco, Top. Curr. Chem., 208, 7 (2000); T. G. Back, Organoselenium Chemistry: A Practical
Approach, Oxford University Press, Oxford, 1999; C. Paulmier, Selenium Reagents and Intermediates
in Organic Chemistry, Pergamon Press, Oxford, 1986; D. Liotta, Organoselenium Chemistry, Wiley,
New York, 1987; S. Patai, ed., The Chemistry of Organic Selenium and Tellurium Compounds, Vols. 1
and 2, Wiley, New York, 1987.