Page 527 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 527
508
CHAPTER 5
Polar Addition 0.52
and Elimination 1.29 1.24
Reactions –0.36 O
O –0.51
1.46 1.89
0.03 1.01
2.03 2.03
0.16
1.37
0.16
(a) ΔE a = 14.1 kcal/mol
0.52 0.52
1.24 1.23
1.29 1.29
O –0.50 O –0.50
–0.36 O –0.35 O
1.72 1.77
1.85 1.82
1.00 1.00
–0.01 O –0.01 O
2.00 2.12 2.05 2.23
0.16 0.39
0.11 0.05
1.37 1.37
0.06 O
(b) ΔE = 12.0 kcal/mol (c) ΔE = 8.5 kcal/mol
a a
0.55
0.53 1.23
1.23
1.29 –0.48
1.29 O
O –0.49
–0.34 O
–0.33 O 1.72
1.78
1.82
1.82
1.00
–0.02 O
1.00
–0.01 O
2.27
1.81
1.92 2.35 0.08
0.10
–0.23 0.37
0.11 1.38
1.38 N –0.45
0.11 –0.01
= 17.3 kcal/mol
(e) ΔE a
(d) ΔE = 11.7 kcal/mol
a
∗
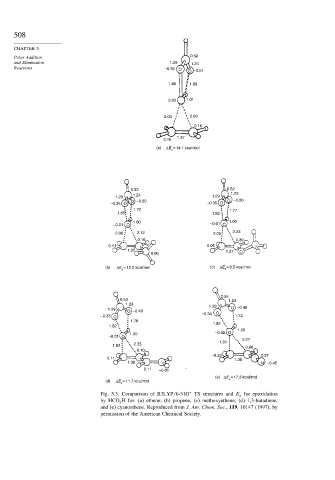
Fig. 5.3. Comparison of B3LYP/6-31G TS structures and E a for epoxidation
by HCO 3 H for: (a) ethene; (b) propene; (c) methoxyethene; (d) 1,3-butadiene,
and (e) cyanoethene. Reproduced from J. Am. Chem. Soc., 119, 10147 (1997), by
permission of the American Chemical Society.