Page 530 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 530
epoxidation. 156 The positively charged quaternary nitrogen enhances the reactivity of 511
the carbonyl group toward nucleophilic addition and also makes the dioxirane inter-
SECTION 5.5
mediate more reactive.
Addition Reactions
CH 3 Involving Epoxides
N + O O
CH 3
PhCH CHCH OH PhCH CHCH OH
2
2
HOOSO K
3
5.5.2. Subsequent Transformations of Epoxides
Epoxides are useful synthetic intermediates and the conversion of an alkene to
an epoxide is often part of a more extensive overall transformation. 157 Advantage is
taken of the reactivity of the epoxide ring to introduce additional functionality. As
epoxide ring opening is usually stereospecific, such reactions can be used to establish
stereochemical relationships between adjacent substituents. Such two- or three-step
operations can achieve specific oxidative transformations of an alkene that might not
be easily accomplished in a single step.
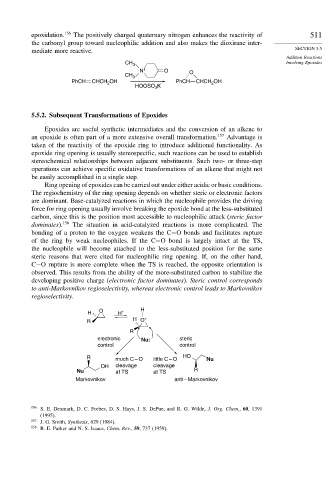
Ring opening of epoxides can be carried out under either acidic or basic conditions.
The regiochemistry of the ring opening depends on whether steric or electronic factors
are dominant. Base-catalyzed reactions in which the nucleophile provides the driving
force for ring opening usually involve breaking the epoxide bond at the less-substituted
carbon, since this is the position most accessible to nucleophilic attack (steric factor
dominates). 158 The situation in acid-catalyzed reactions is more complicated. The
bonding of a proton to the oxygen weakens the C−O bonds and facilitates rupture
of the ring by weak nucleophiles. If the C−O bond is largely intact at the TS,
the nucleophile will become attached to the less-substituted position for the same
steric reasons that were cited for nucleophilic ring opening. If, on the other hand,
C−O rupture is more complete when the TS is reached, the opposite orientation is
observed. This results from the ability of the more-substituted carbon to stabilize the
developing positive charge (electronic factor dominates). Steric control corresponds
to anti-Markovnikov regioselectivity, whereas electronic control leads to Markovnikov
regioselectivity.
H O H + H
H +
R O
R
electronic Nu: steric
control control
R much C – O little C – O HO Nu
OH cleavage cleavage
Nu at TS at TS R
Markovnikov anti – Markovnikov
156 S. E. Denmark, D. C. Forbes, D. S. Hays, J. S. DePue, and R. G. Wilde, J. Org. Chem., 60, 1391
(1995).
157 J. G. Smith, Synthesis, 629 (1984).
158
R. E. Parker and N. S. Isaacs, Chem. Rev., 59, 737 (1959).